The mesoderm is the middle layer of the three germ layers that develops during gastrulation in the very early development of the embryo of most animals. The outer layer is the ectoderm, and the inner layer is the endoderm.

The lens, or crystalline lens, is a transparent biconvex structure in most land vertebrate eyes. Along with the cornea, aqueous and vitreous humours it refracts light, focusing it onto the retina. In many land animals the shape of the lens can be altered, effectively changing the focal length of the eye, enabling them to focus on objects at various distances. This adjustment of the lens is known as accommodation. In many fully aquatic vertebrates such as fish other methods of accommodation are used such as changing the lens's position relative to the retina rather than changing lens shape. Accommodation is analogous to the focusing of a photographic camera via changing its lenses. In land vertebrates the lens is flatter on its anterior side than on its posterior side, while in fish the lens is often close to spherical.

The ectoderm is one of the three primary germ layers formed in early embryonic development. It is the outermost layer, and is superficial to the mesoderm and endoderm. It emerges and originates from the outer layer of germ cells. The word ectoderm comes from the Greek ektos meaning "outside", and derma meaning "skin".
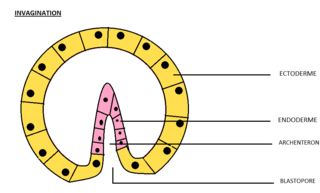
Invagination is the process of a surface folding in on itself to form a cavity, pouch or tube. In developmental biology, invagination is a mechanism that takes place during gastrulation. This mechanism or cell movement happens mostly in the vegetal pole. Invagination consists of the folding of an area of the exterior sheet of cells towards the inside of the blastula. In each organism, the complexity will be different depending on the number of cells. Invagination can be referenced as one of the steps of the establishment of the body plan. The term, originally used in embryology, has been adopted in other disciplines as well.

The olfactory epithelium is a specialized epithelial tissue inside the nasal cavity that is involved in smell. In humans, it measures 5 cm2 (0.78 sq in) and lies on the roof of the nasal cavity about 7 cm (2.8 in) above and behind the nostrils. The olfactory epithelium is the part of the olfactory system directly responsible for detecting odors.

Neurulation refers to the folding process in vertebrate embryos, which includes the transformation of the neural plate into the neural tube. The embryo at this stage is termed the neurula.
A germ layer is a primary layer of cells that forms during embryonic development. The three germ layers in vertebrates are particularly pronounced; however, all eumetazoans produce two or three primary germ layers. Some animals, like cnidarians, produce two germ layers making them diploblastic. Other animals such as bilaterians produce a third layer between these two layers, making them triploblastic. Germ layers eventually give rise to all of an animal's tissues and organs through the process of organogenesis.
Organogenesis is the phase of embryonic development that starts at the end of gastrulation and continues until birth. During organogenesis, the three germ layers formed from gastrulation form the internal organs of the organism.
A neurogenic placode is an area of thickening of the epithelium in the embryonic head ectoderm layer that gives rise to neurons and other structures of the sensory nervous system.
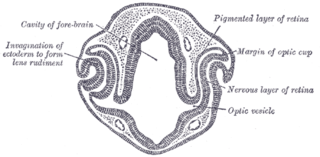
The eyes begin to develop as a pair of diverticula (pouches) from the lateral aspects of the forebrain. These diverticula make their appearance before the closure of the anterior end of the neural tube; after the closure of the tube around the 4th week of development, they are known as the optic vesicles. Previous studies of optic vesicles suggest that the surrounding extraocular tissues – the surface ectoderm and extraocular mesenchyme – are necessary for normal eye growth and differentiation.

Bone morphogenetic protein 4 is a protein that in humans is encoded by BMP4 gene. BMP4 is found on chromosome 14q22-q23.

Mesenchyme is a type of loosely organized animal embryonic connective tissue of undifferentiated cells that give rise to most tissues, such as skin, blood or bone. The interactions between mesenchyme and epithelium help to form nearly every organ in the developing embryo.

Otic vesicle, or auditory vesicle, consists of either of the two sac-like invaginations formed and subsequently closed off during embryonic development. It is part of the neural ectoderm, which will develop into the membranous labyrinth of the inner ear. This labyrinth is a continuous epithelium, giving rise to the vestibular system and auditory components of the inner ear. During the earlier stages of embryogenesis, the otic placode invaginates to produce the otic cup. Thereafter, the otic cup closes off, creating the otic vesicle. Once formed, the otic vesicle will reside next to the neural tube medially, and on the lateral side will be paraxial mesoderm. Neural crest cells will migrate rostral and caudal to the placode.

Human embryonic development or human embryogenesis is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. The eight weeks has 23 stages.

The surface ectoderm, AKA external ectoderm, is one of the two early embryonic divisions of the ectoderm. The other early division of the ectoderm is the neuroectoderm.

The lens placode is a thickened portion of ectoderm that serves as the precursor to the lens.
In embryology, the otic placode is a thickening of the ectoderm on the outer surface of a developing embryo from which the ear develops. The ear, including both the vestibular system and the auditory system, develops from the otic placode beginning the third week of development. During the fourth week, the otic placode invaginates into the mesenchyme adjacent to the rhombencephalon to form the otic pit, which then pinches off from the surface ectoderm to form the otic vesicle.

Retinal precursor cells are biological cells that differentiate into the various cell types of the retina during development. In the vertebrate, these retinal cells differentiate into seven cell types, including retinal ganglion cells, amacrine cells, bipolar cells, horizontal cells, rod photoreceptors, cone photoreceptors, and Müller glia cells. During embryogenesis, retinal cells originate from the anterior portion of the neural plate termed the eye field. Eye field cells with a retinal fate express several transcription factor markers including Rx1, Pax6, and Lhx2. The eye field gives rise to the optic vesicle and then to the optic cup. The retina is generated from the precursor cells within the inner layer of the optic cup, as opposed to the retinal pigment epithelium that originate from the outer layer of the optic cup. In general, the developing retina is organized so that the least-committed precursor cells are located in the periphery of the retina, while the committed cells are located in the center of the retina. The differentiation of retinal precursor cells into the mature cell types found in the retina is coordinated in time and space by factors within the cell as well as factors in the environment of the cell. One example of an intrinsic regulator of this process is the transcription factor Ath5. Ath5 expression in retinal progenitor cells biases their differentiation into a retinal ganglion cell fate. An example of an environmental factor is the morphogen sonic hedge hog (Shh). Shh has been shown to repress the differentiation of precursor cells into retinal ganglion cells.
The face and neck development of the human embryo refers to the development of the structures from the third to eighth week that give rise to the future head and neck. They consist of three layers, the ectoderm, mesoderm and endoderm, which form the mesenchyme, neural crest and neural placodes. The paraxial mesoderm forms structures named somites and somitomeres that contribute to the development of the floor of the brain and voluntary muscles of the craniofacial region. The lateral plate mesoderm consists of the laryngeal cartilages. The three tissue layers give rise to the pharyngeal apparatus, formed by six pairs of pharyngeal arches, a set of pharyngeal pouches and pharyngeal grooves, which are the most typical feature in development of the head and neck. The formation of each region of the face and neck is due to the migration of the neural crest cells which come from the ectoderm. These cells determine the future structure to develop in each pharyngeal arch. Eventually, they also form the neurectoderm, which forms the forebrain, midbrain and hindbrain, cartilage, bone, dentin, tendon, dermis, pia mater and arachnoid mater, sensory neurons, and glandular stroma.
Pax-6, member of the Pax gene class, is responsible for carrying the genetic information that will encode Pax-6 (protein) which dictates the development of the olfactory epithelium, eyes and central nervous system in vertebrates. Pax-6 is expressed as a transcription factor when neural ectoderm receives a combination of weak sonic hedgehog and a strong TGF-Beta signaling gradients. Expression is first seen in the forebrain, hindbrain, head ectoderm and spinal cord followed by later expression in midbrain. Expression in the head ectoderm will give rise to the nasal placodes and to the eye placodes. The nasal placodes will give rise to the olfactory epithelium while eye placodes give rise to the lens and cornea. Expression in the different brain regions is orchestrated with the combinatorial expression of other transcription factors to give rise to the central nervous system. Experiments in mice demonstrate that a deficiency in Pax-6 leads to decrease in brain size, brain structure abnormality leading to Autism, lack of iris formation or a thin cornea. Knockout experiments produced eyeless phenotypes reinforcing the gene’s role in eye development. Advancing research in this area may lead us to better understanding of the complexity seen in neural development and maybe one day be able to grow eye tissue in vitro.