Related Research Articles
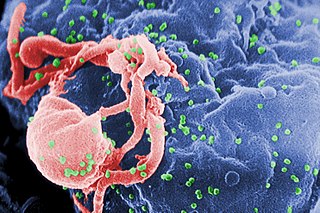
The human immunodeficiency viruses (HIV) are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, the average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.
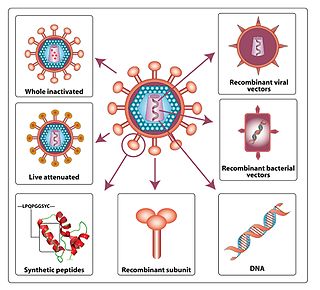
An HIV vaccine is a potential vaccine that could be either a preventive vaccine or a therapeutic vaccine, which means it would either protect individuals from being infected with HIV or treat HIV-infected individuals. It is thought that an HIV vaccine could either induce an immune response against HIV or consist of preformed antibodies against HIV.
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death. HAART also prevents the transmission of HIV between serodiscordant same-sex and opposite-sex partners so long as the HIV-positive partner maintains an undetectable viral load.
The spread of HIV/AIDS has affected millions of people worldwide; AIDS is considered a pandemic. The World Health Organization (WHO) estimated that in 2016 there were 36.7 million people worldwide living with HIV/AIDS, with 1.8 million new HIV infections per year and 1 million deaths due to AIDS. Misconceptions about HIV and AIDS arise from several different sources, from simple ignorance and misunderstandings about scientific knowledge regarding HIV infections and the cause of AIDS to misinformation propagated by individuals and groups with ideological stances that deny a causative relationship between HIV infection and the development of AIDS. Below is a list and explanations of some common misconceptions and their rebuttals.

Simian immunodeficiency virus (SIV) is a species of retrovirus that cause persistent infections in at least 45 species of non-human primates. Based on analysis of strains found in four species of monkeys from Bioko Island, which was isolated from the mainland by rising sea levels about 11,000 years ago, it has been concluded that SIV has been present in monkeys and apes for at least 32,000 years, and probably much longer.

Human T-cell lymphotropic virus type 1 or human T-lymphotropic virus (HTLV-I), also called the adult T-cell lymphoma virus type 1, is a retrovirus of the human T-lymphotropic virus (HTLV) family.
Coinfection is the simultaneous infection of a host by multiple pathogen species. In virology, coinfection includes simultaneous infection of a single cell by two or more virus particles. An example is the coinfection of liver cells with hepatitis B virus and hepatitis D virus, which can arise incrementally by initial infection followed by superinfection.
A superinfection is a second infection superimposed on an earlier one, especially by a different microbial agent of exogenous or endogenous origin, that is resistant to the treatment being used against the first infection. Examples of this in bacteriology are the overgrowth of endogenous Clostridioides difficile that occurs following treatment with a broad-spectrum antibiotic, and pneumonia or sepsis from Pseudomonas aeruginosa in some immunocompromised patients.

Feline immunodeficiency virus (FIV) is a Lentivirus that affects cats worldwide, with 2.5% to 4.4% of felines being infected.
Modified vaccinia Ankara (MVA) is an attenuated (weakened) strain of the vaccinia virus. It is being used as a vaccine against smallpox and mpox, having fewer side effects than smallpox vaccines derived from other poxviruses.
Following infection with HIV-1, the rate of clinical disease progression varies between individuals. Factors such as host susceptibility, genetics and immune function, health care and co-infections as well as viral genetic variability may affect the rate of progression to the point of needing to take medication in order not to develop AIDS.
The genome and proteins of HIV (human immunodeficiency virus) have been the subject of extensive research since the discovery of the virus in 1983. "In the search for the causative agent, it was initially believed that the virus was a form of the Human T-cell leukemia virus (HTLV), which was known at the time to affect the human immune system and cause certain leukemias. However, researchers at the Pasteur Institute in Paris isolated a previously unknown and genetically distinct retrovirus in patients with AIDS which was later named HIV." Each virion comprises a viral envelope and associated matrix enclosing a capsid, which itself encloses two copies of the single-stranded RNA genome and several enzymes. The discovery of the virus itself occurred two years following the report of the first major cases of AIDS-associated illnesses.
HIV tropism refers to the cell type in which the human immunodeficiency virus (HIV) infects and replicates. HIV tropism of a patient's virus is measured by the Trofile assay.
Visna-maedi virus from the genus Lentivirus and subfamily Orthoretrovirinae, is a retrovirus that causes encephalitis and chronic pneumonitis in sheep. It is known as visna when found in the brain, and maedi when infecting the lungs. Lifelong, persistent infections in sheep occur in the lungs, lymph nodes, spleen, joints, central nervous system, and mammary glands; The condition is sometimes known as ovine progressive pneumonia (OPP), particularly in the United States, or Montana sheep disease. White blood cells of the monocyte/macrophage lineage are the main target of the virus.

The subtypes of HIV include two main subtypes, known as HIV type 1 (HIV-1) and HIV type 2 (HIV-2). These subtypes have distinct genetic differences and are associated with different epidemiological patterns and clinical characteristics.
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy.
MVA-B, or Modified Vaccinia Ankara B, is an HIV vaccine created to give immune resistance to infection by the human immunodeficiency virus. It was developed by a team of Spanish researchers at the Spanish National Research Council's Biotechnology National Centre headed by Dr. Mariano Esteban. The vaccine is based on the Modified vaccinia Ankara (MVA) virus used during the 1970s to help eradicate the smallpox virus. The B in the name "refers to HIV-B, the most common HIV subtype in Europe". It has been stated by Dr. Esteban that, in the future, the vaccine could potentially reduce the virulence of HIV to a "minor chronic infection akin to herpes".
SAV001-H is the first candidate preventive HIV vaccine using a killed or "dead" version of the HIV-1 virus.

The stages of HIV infection are acute infection, latency, and AIDS. Acute infection lasts for several weeks and may include symptoms such as fever, swollen lymph nodes, inflammation of the throat, rash, muscle pain, malaise, and mouth and esophageal sores. The latency stage involves few or no symptoms and can last anywhere from two weeks to twenty years or more, depending on the individual. AIDS, the final stage of HIV infection, is defined by low CD4+ T cell counts, various opportunistic infections, cancers, and other conditions.

HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
References
- 1 2 3 4 5 Smith DM, Strain MC, Frost SD, Pillai SK, Wong JK, Wrin T, Liu Y, Petropolous CJ, Daar ES, Little SJ, Richman DD (November 2006). "Lack of neutralizing antibody response to HIV-1 predisposes to superinfection". Virology. 355 (1): 1–5. doi: 10.1016/j.virol.2006.08.009 . PMID 16962152.
- 1 2 3 4 5 Redd AD, Mullis CE, Serwadda D, Kong X, Martens C, Ricklefs SM, Tobian AA, Xiao C, Grabowski MK, Nalugoda F, Kigozi G, Laeyendecker O, Kagaayi J, Sewankambo N, Gray RH, Porcella SF, Wawer MJ, Quinn TC (July 2012). "The rates of HIV superinfection and primary HIV incidence in a general population in Rakai, Uganda". The Journal of Infectious Diseases. 206 (2): 267–74. doi:10.1093/infdis/jis325. PMC 3415936 . PMID 22675216.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 Redd AD, Quinn TC, Tobian AA (July 2013). "Frequency and implications of HIV superinfection". The Lancet. Infectious Diseases. 13 (7): 622–8. doi:10.1016/s1473-3099(13)70066-5. PMC 3752600 . PMID 23726798.
- ↑ Foley B.T. (2017). HIV and SIV Evolution. In: Shapshak P. et al. (eds) Global Virology II - HIV and NeuroAIDS. doi:10.1007/978-1-4939-7290-6_5.
- 1 2 3 Piantadosi A, Chohan B, Chohan V, McClelland RS, Overbaugh J (November 2007). "Chronic HIV-1 infection frequently fails to protect against superinfection". PLOS Pathogens. 3 (11): e177. doi: 10.1371/journal.ppat.0030177 . PMC 2077901 . PMID 18020705.
- ↑ Blish CA, Dogan OC, Jaoko W, McClelland RS, Mandaliya K, Odem-Davis KS, Richardsonb BA, Overbaugh J (March 2012). "Cellular immune responses and susceptibility to HIV-1 superinfection: a case-control study". AIDS. 26 (5): 643–6. doi:10.1097/QAD.0b013e3283509a0b. PMC 3511787 . PMID 22210637.
- ↑ Cortez V, Odem-Davis K, McClelland RS, Jaoko W, Overbaugh J (2012). "HIV-1 superinfection in women broadens and strengthens the neutralizing antibody response". PLOS Pathogens. 8 (3): e1002611. doi: 10.1371/journal.ppat.1002611 . PMC 3315492 . PMID 22479183.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Smith DM, Richman DD, Little SJ (August 2005). "HIV superinfection". The Journal of Infectious Diseases. 192 (3): 438–44. doi: 10.1086/431682 . PMID 15995957.
- 1 2 3 4 Streeck H, Li B, Poon AF, Schneidewind A, Gladden AD, Power KA, Daskalakis D, Bazner S, Zuniga R, Brander C, Rosenberg ES, Frost SD, Altfeld M, Allen TM (August 2008). "Immune-driven recombination and loss of control after HIV superinfection". The Journal of Experimental Medicine. 205 (8): 1789–96. doi:10.1084/jem.20080281. PMC 2525594 . PMID 18625749.
- 1 2 3 4 5 6 Altfeld M, Allen TM, Yu XG, Johnston MN, Agrawal D, Korber BT, Montefiori DC, O'Connor DH, Davis BT, Lee PK, Maier EL, Harlow J, Goulder PJ, Brander C, Rosenberg ES, Walker BD (November 2002). "HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus". Nature. 420 (6914): 434–9. Bibcode:2002Natur.420..434A. doi: 10.1038/nature01200 . PMID 12459786. S2CID 52859094.
- 1 2 3 4 Smith DM, Wong JK, Hightower GK, Ignacio CC, Koelsch KK, Petropoulos CJ, Richman DD, Little SJ (August 2005). "HIV drug resistance acquired through superinfection". AIDS. 19 (12): 1251–6. doi: 10.1097/01.aids.0000180095.12276.ac . PMID 16052079. S2CID 1267726.
- ↑ Fang G, Weiser B, Kuiken C, Philpott SM, Rowland-Jones S, Plummer F, Kimani J, Shi B, Kaul R, Bwayo J, Anzala O, Burger H (January 2004). "Recombination following superinfection by HIV-1". AIDS. 18 (2): 153–9. doi: 10.1097/00002030-200401230-00003 . PMID 15075531. S2CID 24770809.
- ↑ "Method of the Year". Nature Methods. 5 (1): 1. January 2008. doi: 10.1038/nmeth1153 . PMID 18175409.
- 1 2 3 4 5 6 Marcus J, McConnel JJ, Grant RM (2005). "HIV Superinfection vs Dual Initial Infection: What Clinicians and Patients Should Know". Medscape HIV/AIDS. 11 (1): 33.
- ↑ Yerly S, Jost S, Monnat M, Telenti A, Cavassini M, Chave JP, Kaiser L, Burgisser P, Perrin L (July 2004). "HIV-1 co/super-infection in intravenous drug users". AIDS. 18 (10): 1413–21. doi: 10.1097/01.aids.0000131330.28762.0c . PMID 15199317. S2CID 24853737.
- 1 2 Campbell MS, Gottlieb GS, Hawes SE, Nickle DC, Wong KG, Deng W, Lampinen TM, Kiviat NB, Mullins JI (May 2009). "HIV-1 superinfection in the antiretroviral therapy era: are seroconcordant sexual partners at risk?". PLOS ONE. 4 (5): e5690. Bibcode:2009PLoSO...4.5690C. doi: 10.1371/journal.pone.0005690 . PMC 2684644 . PMID 19479055.
- 1 2 Taylor JE, Korber BT (January 2005). "HIV-1 intra-subtype superinfection rates: estimates using a structured coalescent with recombination". Infection, Genetics and Evolution. 5 (1): 85–95. doi:10.1016/j.meegid.2004.07.001. PMID 15567142.
- ↑ Fultz PN, Srinivasan A, Greene CR, Butler D, Swenson RB, McClure HM (December 1987). "Superinfection of a chimpanzee with a second strain of human immunodeficiency virus". Journal of Virology. 61 (12): 4026–9. doi:10.1128/JVI.61.12.4026-4029.1987. PMC 256026 . PMID 2446009.
- ↑ Le Guern M, Levy JA (January 1992). "Human immunodeficiency virus (HIV) type 1 can superinfect HIV-2-infected cells: pseudotype virions produced with expanded cellular host range". Proceedings of the National Academy of Sciences of the United States of America. 89 (1): 363–7. Bibcode:1992PNAS...89..363L. doi: 10.1073/pnas.89.1.363 . JSTOR 2358537. PMC 48237 . PMID 1346069.
- ↑ Otten RA, Ellenberger DL, Adams DR, Fridlund CA, Jackson E, Pieniazek D, Rayfield MA (September 1999). "Identification of a window period for susceptibility to dual infection with two distinct human immunodeficiency virus type 2 isolates in a Macaca nemestrina (pig-tailed macaque) model". The Journal of Infectious Diseases. 180 (3): 673–84. doi: 10.1086/314968 . PMID 10438354.
- 1 2 3 Ramos A, Hu DJ, Nguyen L, Phan KO, Vanichseni S, Promadej N, Choopanya K, Callahan M, Young NL, McNicholl J, Mastro TD, Folks TM, Subbarao S (August 2002). "Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users". Journal of Virology. 76 (15): 7444–52. doi:10.1128/JVI.76.15.7444-7452.2002. PMC 136380 . PMID 12097556.
- ↑ Koelsch KK, Smith DM, Little SJ, Ignacio CC, Macaranas TR, Brown AJ, Petropoulos CJ, Richman DD, Wong JK (May 2003). "Clade B HIV-1 superinfection with wild-type virus after primary infection with drug-resistant clade B virus". AIDS. 17 (7): F11-6. doi: 10.1097/00002030-200305020-00001 . PMID 12700477. S2CID 30023240.
