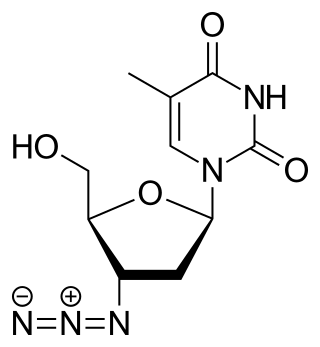
Zidovudine (ZDV), also known as azidothymidine (AZT), is an antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use in combination with other antiretrovirals. It may be used to prevent mother-to-child spread during birth or after a needlestick injury or other potential exposure. It is sold both by itself and together as lamivudine/zidovudine and abacavir/lamivudine/zidovudine. It can be used by mouth or by slow injection into a vein.
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death. HAART also prevents the transmission of HIV between serodiscordant same-sex and opposite-sex partners so long as the HIV-positive partner maintains an undetectable viral load.

Stavudine (d4T), sold under the brand name Zerit among others, is an antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. However, it is not a first-line treatment. It is given by mouth.
Post-exposure prophylaxis, also known as post-exposure prevention (PEP), is any preventive medical treatment started after exposure to a pathogen in order to prevent the infection from occurring.
The National Institute of Allergy and Infectious Diseases is one of the 27 institutes and centers that make up the National Institutes of Health (NIH), an agency of the United States Department of Health and Human Services (HHS). NIAID's mission is to conduct basic and applied research to better understand, treat, and prevent infectious, immunologic, and allergic diseases.

Nevirapine (NVP), sold under the brand name Viramune among others, is a medication used to treat and prevent HIV/AIDS, specifically HIV-1. It is generally recommended for use with other antiretroviral medications. It may be used to prevent mother to child spread during birth but is not recommended following other exposures. It is taken by mouth.
A vertically transmitted infection is an infection caused by pathogenic bacteria or viruses that use mother-to-child transmission, that is, transmission directly from the mother to an embryo, fetus, or baby during pregnancy or childbirth. It can occur when the mother has a pre-existing disease or becomes infected during pregnancy. Nutritional deficiencies may exacerbate the risks of perinatal infections. Vertical transmission is important for the mathematical modelling of infectious diseases, especially for diseases of animals with large litter sizes, as it causes a wave of new infectious individuals.
Christine Joy Maggiore was an HIV-positive activist and promoter of HIV/AIDS denialism. She was the founder of Alive & Well AIDS Alternatives, an organization which disputes the link between HIV and AIDS and urges HIV-positive pregnant women to avoid anti-HIV medication. Maggiore authored and self-published the book What If Everything You Thought You Knew about AIDS Was Wrong?

Emtricitabine/tenofovir, sold under the brand name Truvada among others, is a fixed-dose combination antiretroviral medication used to treat and prevent HIV/AIDS. It contains the antiretroviral medications emtricitabine and tenofovir disoproxil. For treatment, it must be used in combination with other antiretroviral medications. For prevention before exposure, in those who are at high risk, it is recommended along with safer sex practices. It does not cure HIV/AIDS. Emtricitabine/tenofovir is taken by mouth.
The Division of Acquired Immunodeficiency Syndrome (DAIDS) is a division of the National Institute of Allergy and Infectious Diseases, which is part of the National Institutes of Health. It was formed in 1986 as a part of the initiative to address the national research needs created by the advent and spread of the HIV/AIDS epidemic. Specifically, the Division's mission is to increase basic knowledge of the pathogenesis, natural history, and transmission of HIV disease and to support research that promotes progress in its detection, treatment, and prevention. DAIDS accomplishes this through planning, implementing, managing, and evaluating programs in (1) fundamental basic research, (2) discovery and development of therapies for HIV infection and its complications, and (3) discovery and development of vaccines and other prevention strategies.

Infection with HIV, a retrovirus, can be managed with treatment but without treatment can lead to a spectrum of conditions including AIDS.
Human Immunodeficiency Virus (HIV) and Hepatitis C Virus (HCV) co-infection is a multi-faceted, chronic condition that significantly impacts public health. According to the World Health Organization (WHO), 2 to 15% of those infected with HIV are also affected by HCV, increasing their risk of morbidity and mortality due to accelerated liver disease. The burden of co-infection is especially high in certain high-risk groups, such as intravenous drug users and men who have sex with men. These individuals who are HIV-positive are commonly co-infected with HCV due to shared routes of transmission including, but not limited to, exposure to HIV-positive blood, sexual intercourse, and passage of the Hepatitis C virus from mother to infant during childbirth.
The history of HIV/AIDS in Australia is distinctive, as Australian government bodies recognised and responded to the AIDS pandemic relatively swiftly, with the implementation of effective disease prevention and public health programs, such as needle and syringe programs (NSPs). As a result, despite significant numbers of at-risk group members contracting the virus in the early period following its discovery, Australia achieved and has maintained a low rate of HIV infection in comparison to the rest of the world.
HIV prevention refers to practices that aim to prevent the spread of the human immunodeficiency virus (HIV). HIV prevention practices may be undertaken by individuals to protect their own health and the health of those in their community, or may be instituted by governments and community-based organizations as public health policies.
The Mississippi baby is a Mississippi girl who in 2013 was thought to have been cured of HIV. She had contracted HIV at birth from her HIV-positive mother. Thirty hours after the baby was born, she was treated with intense antiretroviral therapy. When the baby was about 18 months old, the mother did not bring the child in for scheduled examinations for the next five months. When the mother returned with the child, doctors expected to find high levels of HIV, but instead the HIV levels were undetectable. The Mississippi baby was thought to be the other person, after the "Berlin patient," to have been cured of HIV. As a result, the National Institutes of Health planned to conduct a worldwide study on aggressive antiretroviral treatment of newborn infants of mothers with HIV infections. It was thought that aggressive antiretroviral therapy on newborn infants might be a cure for HIV. On July 10, 2014, however, it was reported that the child was found to be infected with HIV.
Breastfeeding by HIV-infected mothers is the practice of breastfeeding of HIV-infected mothers and include those who may want to or are currently breastfeeding. HIV can be transmitted to the infant through breastfeeding. The risk of transmission varies and depends on the viral load in the mother's milk. An infant can be infected with HIV throughout the duration of the pregnancy or during childbirth (intrapartum).
Treatment as prevention (TasP) is a concept in public health that promotes treatment as a way to prevent and reduce the likelihood of HIV illness, death and transmission from an infected individual to others. Expanding access to earlier HIV diagnosis and treatment as a means to address the global epidemic by preventing illness, death and transmission was first proposed in 2000 by Garnett et al. The term is often used to talk about treating people that are currently living with human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS) to prevent illness, death and transmission. Although some experts narrow this to only include preventing infections, treatment prevents illnesses such as tuberculosis and has been shown to prevent death. The dual impact on well-being and its 100% effectiveness in reducing transmission makes TasP the most important element in the HIV prevention toolkit. In relation to HIV, antiretroviral therapy (ART) is a three or more drug combination therapy that is used to decrease the viral load, or the measured amount of virus, in an infected individual. Such medications are used as a preventative for infected individuals to not only spread the HIV virus to their negative partners but also improve their current health to increase their lifespans. Other names for ART include highly active antiretroviral therapy (HAART), combination antiretroviral therapy (cART), triple therapy and triple drug cocktail. When taken correctly, ART is able to diminish the presence of the HIV virus in the bodily fluids of an infected person to a level of undetectability. Undetectability ensures that infection does not necessarily have an effect on a person's general health, and that there is no longer a risk of passing along HIV to others. Consistent adherence to an ARV regimen, monitoring, and testing are essential for continued confirmed viral suppression. Treatment as prevention rose to great prominence in 2011, as part of the HPTN 052 study, which shed light on the benefits of early treatment for HIV positive individuals.

Neonatal infections are infections of the neonate (newborn) acquired during prenatal development or within the first four weeks of life. Neonatal infections may be contracted by mother to child transmission, in the birth canal during childbirth, or after birth. Neonatal infections may present soon after delivery, or take several weeks to show symptoms. Some neonatal infections such as HIV, hepatitis B, and malaria do not become apparent until much later. Signs and symptoms of infection may include respiratory distress, temperature instability, irritability, poor feeding, failure to thrive, persistent crying and skin rashes.

Janet L. Mitchell was an American physician known for her advances in perinatal HIV/AIDS treatment. During the early days of the AIDS epidemic in the U.S. Mitchell developed protocols for health treatment of pregnant women who were HIV positive or at risk for developing AIDS. She advocated against mandatory testing and testifying before Congress, she advocated in favor of an inclusive approach to health care and social services. One of her innovations derived from a study that saw a 70% decrease in HIV transmission to babies when AZT was administered to their mothers during the pregnancy.
Viral load monitoring for HIV is the regular measurement of the viral load of individual HIV-positive people as part of their personal plan for treatment of HIV/AIDS. A count of the viral load is routine before the start of HIV treatment.