
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.

A polymer is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, cups, jars, etc.). As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, and thermoforming for manufacturing, and in combination with glass fibre for engineering resins.
Amorphous poly alpha olefin is a commodity chemical used in multiple applications.
Polyamide-imides are either thermosetting or thermoplastic, amorphous polymers that have exceptional mechanical, thermal and chemical resistant properties. Polyamide-imides are used extensively as wire coatings in making magnet wire. They are prepared from isocyanates and TMA in N-methyl-2-pyrrolidone (NMP). A prominent distributor of polyamide-imides is Solvay Specialty Polymers, which uses the trademark Torlon.

Ethylene-vinyl acetate (EVA), also known as poly(ethylene-vinyl acetate) (PEVA), is a copolymer of ethylene and vinyl acetate. The weight percent of vinyl acetate usually varies from 10 to 50%, with the remainder being ethylene. There are three different types of EVA copolymer, which differ in the vinyl acetate (VA) content and the way the materials are used.

Polyphthalamide is a subset of thermoplastic synthetic resins in the polyamide (nylon) family defined as when 55% or more moles of the carboxylic acid portion of the repeating unit in the polymer chain is composed of a combination of terephthalic (TPA) and isophthalic (IPA) acids. The substitution of aliphatic diacids by aromatic diacids in the polymer backbone increases the melting point, glass transition temperature, chemical resistance and stiffness.

Fluorinated ethylene propylene (FEP) is a copolymer of hexafluoropropylene and tetrafluoroethylene. It differs from the polytetrafluoroethylene (PTFE) resins in that it is melt-processable using conventional injection molding and screw extrusion techniques. Fluorinated ethylene propylene was invented by DuPont and is sold under the brandname Teflon FEP. Other brandnames are Neoflon FEP from Daikin or Dyneon FEP from Dyneon/3M.

Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
Wood glue is an adhesive used to tightly bond pieces of wood together. Many substances have been used as glues. Traditionally animal proteins like casein from milk or collagen from animal hides and bones were boiled down to make early glues. They worked by solidifying as they dried. Later, glues were made from plant starches like flour or potato starch. When combined with water and heated, the starch gelatinizes and forms a sticky paste as it dries. Plant-based glues were common for books and paper products, though they can break down more easily over time compared to animal-based glues. Examples of modern wood glues include polyvinyl acetate (PVA) and epoxy resins. Some resins used in producing composite wood products may contain formaldehyde. As of 2021, “the wood panel industry uses almost 95% of synthetic petroleum-derived thermosetting adhesives, mainly based on urea, phenol, and melamine, among others”.

Pressure-sensitive adhesive is a type of nonreactive adhesive which forms a bond when pressure is applied to bond the adhesive with a surface. No solvent, water, or heat is needed to activate the adhesive. It is used in pressure-sensitive tapes, labels, glue dots, stickers, sticky note pads, automobile trim, and a wide variety of other products.
Polyester resins are synthetic resins formed by the reaction of dibasic organic acids and polyhydric alcohols. Maleic anhydride is a commonly used raw material with diacid functionality in unsaturated polyester resins. Unsaturated polyester resins are used in sheet moulding compound, bulk moulding compound and the toner of laser printers. Wall panels fabricated from polyester resins reinforced with fiberglass—so-called fiberglass reinforced plastic (FRP)—are typically used in restaurants, kitchens, restrooms and other areas that require washable low-maintenance walls. They are also used extensively in cured-in-place pipe applications. Departments of Transportation in the USA also specify them for use as overlays on roads and bridges. In this application they are known AS Polyester Concrete Overlays (PCO). These are usually based on isophthalic acid and cut with styrene at high levels—usually up to 50%. Polyesters are also used in anchor bolt adhesives though epoxy based materials are also used. Many companies have and continue to introduce styrene free systems mainly due to odor issues, but also over concerns that styrene is a potential carcinogen. Drinking water applications also prefer styrene free. Most polyester resins are viscous, pale coloured liquids consisting of a solution of a polyester in a reactive diluent which is usually styrene, but can also include vinyl toluene and various acrylates.
Tackifiers are chemical compounds used in formulating adhesives to increase tack, the stickiness of the surface of the adhesive. They are usually low-molecular weight compounds with high glass transition temperature. At low strain rate, they provide higher stress compliance, and become stiffer at higher strain rates.
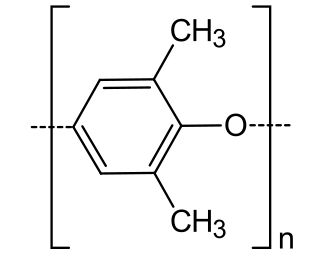
Poly(p-phenylene oxide) (PPO), poly(p-phenylene ether) (PPE), poly(oxy-2,6-dimethyl-1,4-phenylene), often referred to simply as polyphenylene oxide, is a high-temperature thermoplastic with the general formula (C8H8O)n. It is rarely used in its pure form due to difficulties in processing. It is mainly used as blend with polystyrene, high impact styrene-butadiene copolymer or polyamide. PPO is a registered trademark of SABIC Innovative Plastics B.V. under which various polyphenylene ether resins are sold.
Low pressure molding (LPM) with polyamide and polyolefin (hot-melt) materials is a process typically used to encapsulate and environmentally protect electronic components. The purpose is to protect electronics against moisture, dust dirt and vibration. Low pressure molding is also used for sealing connectors and molding grommets and strain reliefs.
The chemistry of pressure-sensitive adhesives describes the chemical science associated with pressure-sensitive adhesives (PSA). PSA tapes and labels have become an important part of everyday life. These rely on adhesive material affixed to a backing such as paper or plastic film.
Hot melt coating is the application of a layer to a substrate by pre-melting the desired material and then allowing or forcing the material to cool, solidifying the layer. The process is widely used in industry, including pressure-sensitive adhesives, labels, pharmaceuticals, etc.
Adhesive bonding is a joining technique used in the manufacture and repair of a wide range of products. Along with welding and soldering, adhesive bonding is one of the basic joining processes. In this technique, components are bonded together using adhesives. The broad range of types of adhesives available allows numerous materials to be bonded together in products as diverse as vehicles, mobile phones, personal care products, buildings, computers and medical devices.











