Related Research Articles

Cyclic voltammetry (CV) is a type of potentiodynamic electrochemical measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.
Coulometry determines the amount of matter transformed during an electrolysis reaction by measuring the amount of electricity consumed or produced. It can be used for precision measurements of charge, and the amperes even used to have a coulometric definition. However, today coulometry is mainly used for analytical applications. Coulometry is a group of techniques in analytical chemistry. It is named after Charles-Augustin de Coulomb.
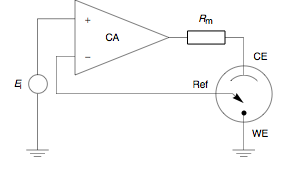
A potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments. A Bipotentiostat and polypotentiostat are potentiostats capable of controlling two working electrodes and more than two working electrodes, respectively.

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammagram which plots the current produced by the analyte versus the potential of the working electrode.

Chronoamperometry is an electrochemical technique in which the potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells this decay is much slower than the charging decay-cells with no supporting electrolyte are notable exceptions. Most commonly a three electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal to noise ratio in comparison to other amperometric techniques.
Polarography is a type of voltammetry where the working electrode is a dropping mercury electrode (DME) or a static mercury drop electrode (SMDE), which are useful for their wide cathodic ranges and renewable surfaces. It was invented in 1922 by Czech chemist Jaroslav Heyrovský, for which he won the Nobel prize in 1959.
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The three main categories are potentiometry, coulometry, and voltammetry.

Linear sweep voltammetry is a voltammetric method where the current at a working electrode is measured while the potential between the working electrode and a reference electrode is swept linearly in time. Oxidation or reduction of species is registered as a peak or trough in the current signal at the potential at which the species begins to be oxidized or reduced.
The working electrode is the electrode in an electrochemical system on which the reaction of interest is occurring. The working electrode is often used in conjunction with an auxiliary electrode, and a reference electrode in a three electrode system. Depending on whether the reaction on the electrode is a reduction or an oxidation, the working electrode is called cathodic or anodic, respectively. Common working electrodes can consist of materials ranging from inert metals such as gold, silver or platinum, to inert carbon such as glassy carbon, boron doped diamond or pyrolytic carbon, and mercury drop and film electrodes. Chemically modified electrodes are employed for the analysis of both organic and inorganic samples.
Squarewave voltammetry (SWV) is a form of linear potential sweep voltammetry that uses a combined square wave and staircase potential applied to a stationary electrode. It has found numerous applications in various fields, including within medicinal and various sensing communities.
The auxiliary electrode, often also called the counter electrode, is an electrode used in a three electrode electrochemical cell for voltammetric analysis or other reactions in which an electric current is expected to flow. The auxiliary electrode is distinct from the reference electrode, which establishes the electrical potential against which other potentials may be measured, and the working electrode, at which the cell reaction takes place.
In chemistry, an electrochemical reaction mechanism is the step by step sequence of elementary steps, involving at least one outer sphere electron transfer, by which an overall chemical change occurs.
A rotating disk electrode (RDE) is a hydrodynamic working electrode used in a three electrode system. The electrode rotates during experiments inducing a flux of analyte to the electrode. These working electrodes are used in electrochemical studies when investigating reaction mechanisms related to redox chemistry, among other chemical phenomena. The more complex rotating ring-disk electrode can be used as a rotating disk electrode if the ring is left inactive during the experiment.
A rotating ring-disc electrode (RRDE) is a double working electrode used in hydrodynamic voltammetry, very similar to a rotating disk electrode (RDE). The electrode rotates during experiments inducing a flux of analyte to the electrode. This system used in electrochemical studies when investigating reaction mechanisms related to redox chemistry and other chemical phenomena.
An ultramicroelectrode (UME) is a working electrode used in a voltammetry. The small size of UME give them large diffusion layers and small overall currents. These features allow UME to achieve useful steady-state conditions and very high scan rates (V/s) with limited distortion. UME were developed independently by Wightman and Fleischmann around 1980. Small current at UME enables electrochemical measurements in low conductive media, where voltage drop associated with high solution resistance makes these experiments difficult for conventional electrodes. Furthermore, small voltage drop at UME leads to a very small voltage distortion at the electrode-solution interface which allows using two-electrode setup in voltammetric experiment instead of conventional three-electrode setup.

The dropping mercury electrode (DME) is a working electrode made of mercury and used in polarography. Experiments run with mercury electrodes are referred to as forms of polarography even if the experiments are identical or very similar to a corresponding voltammetry experiment which uses solid working electrodes. Like other working electrodes these electrodes are used in electrochemical studies using three electrode systems when investigating reaction mechanisms related to redox chemistry among other chemical phenomena.

The hanging mercury drop electrode is a working electrode variation on the dropping mercury electrode (DME). Experiments run with dropping mercury electrodes are referred to as forms of polarography. If the experiments are performed at an electrode with a constant surface it is referred as voltammetry.
Bulk electrolysis is also known as potentiostatic coulometry or controlled potential coulometry. The experiment is a form of coulometry which generally employs a three electrode system controlled by a potentiostat. In the experiment the working electrode is held at a constant potential (volts) and current (amps) is monitored over time (seconds). In a properly run experiment an analyte is quantitatively converted from its original oxidation state to a new oxidation state, either reduced or oxidized. As the substrate is consumed, the current also decreases, approaching zero when the conversion nears completion.
Scanning electrochemical microscopy (SECM) is a technique within the broader class of scanning probe microscopy (SPM) that is used to measure the local electrochemical behavior of liquid/solid, liquid/gas and liquid/liquid interfaces. Initial characterization of the technique was credited to University of Texas electrochemist, Allen J. Bard, in 1989. Since then, the theoretical underpinnings have matured to allow widespread use of the technique in chemistry, biology and materials science. Spatially resolved electrochemical signals can be acquired by measuring the current at an ultramicroelectrode (UME) tip as a function of precise tip position over a substrate region of interest. Interpretation of the SECM signal is based on the concept of diffusion-limited current. Two-dimensional raster scan information can be compiled to generate images of surface reactivity and chemical kinetics.
Ultraviolet-visible (UV-Vis) absorption spectroelectrochemistry (SEC) is a multiresponse technique that analyzes the evolution of the absorption spectra in UV-Vis regions during an electrode process. This technique provides information from an electrochemical and spectroscopic point of view. In this way, it enables a better perception about the chemical system of interest. On one hand, molecular information related to the electronic levels of the molecules is obtained from the evolution of the spectra. On the other hand, kinetic and thermodynamic information of the processes is obtained from the electrochemical signal.
References
- ↑ Douglas A. Skoog; F. James Holler; Stanley R. Crouch (27 January 2017). Principles of Instrumental Analysis. Cengage Learning. pp. 660–. ISBN 978-1-305-57721-3.
- ↑ Richard G. Compton; Craig E. Banks (2011). Understanding Voltammetry. World Scientific. pp. 427–. ISBN 978-1-84816-586-1.
- ↑ Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications. New York: John Wiley & Sons, 2nd Edition, 2000 ISBN 0-471-40521-3.
- ↑ Cynthia G. Zoski (Editor) Handbook of Electrochemistry. Elsevier, 2007 ISBN 0-444-51958-0
- ↑ Peter T. Kissinger, William R. Heineman Laboratory Techniques in Electroanalytical Chemistry. CRC Press, 1996 ISBN 0-8247-9445-1
- ↑ Douglas A. Skoog, F. James Holler, Timothy A. Nieman Principles of Instrumental Analysis. Harcourt Brace College Publishers, 1998 ISBN 0-03-002078-6.