Catalysis is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst. Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process regenerating the catalyst.

Hydrogen peroxide is a chemical compound with the formula H
2O
2. In its pure form, it is a very pale blue liquid, slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or "high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry.
Hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol. The process results in the syn addition of a hydrogen and a hydroxyl group where the double bond had been. Hydroboration–oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. The reaction thus provides a more stereospecific and complementary regiochemical alternative to other hydration reactions such as acid-catalyzed addition and the oxymercuration–reduction process. The reaction was first reported by Herbert C. Brown in the late 1950s and it was recognized in his receiving the Nobel Prize in Chemistry in 1979.

An epoxide is a cyclic ether with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.

Methyl violet is a family of organic compounds that are mainly used as dyes. Depending on the number of attached methyl groups, the color of the dye can be altered. Its main use is as a purple dye for textiles and to give deep violet colors in paint and ink, it is also used as a hydration indicator for silica gel. Methyl violet 10B is also known as crystal violet and has medical uses.
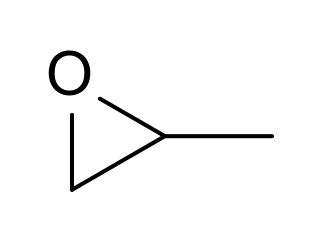
Propylene oxide is an acutely toxic and carcinogenic organic compound with the molecular formula CH3CHCH2O. This colorless volatile liquid with an odor similar to ether, is produced on a large scale industrially. Its major application is its use for the production of polyether polyols for use in making polyurethane plastics. It is a chiral epoxide, although it is commonly used as a racemic mixture.
Fenton's reagent is a solution of hydrogen peroxide (H2O2) with ferrous iron (typically iron(II) sulfate, FeSO4) as a catalyst that is used to oxidize contaminants or waste waters. Fenton's reagent can be used to destroy organic compounds such as trichloroethylene (TCE) and tetrachloroethylene (perchloroethylene, PCE). It was developed in the 1890s by Henry John Horstman Fenton as an analytical reagent.

In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalyzed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity (PCA) depends on the ability of the catalyst to create electron–hole pairs, which generate free radicals (e.g. hydroxyl radicals: •OH) able to undergo secondary reactions. Its practical application was made possible by the discovery of water electrolysis by means of titanium dioxide (TiO2).

Sodium sulfide is the chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts are colorless solids. They are water-soluble, giving strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, an extremely toxic, flammable and corrosive gas which smells like rotten eggs.
Organoselenium compounds are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
Nanomaterial-based catalysts are usually heterogeneous catalysts broken up into metal nanoparticles in order to enhance the catalytic process. Metal nanoparticles have high surface area, which can increase catalytic activity. Nanoparticle catalysts can be easily separated and recycled. They are typically used under mild conditions to prevent decomposition of the nanoparticles.
Advanced oxidation processes (AOPs), in a broad sense, are a set of chemical treatment procedures designed to remove organic (and sometimes inorganic) materials in water and wastewater by oxidation through reactions with hydroxyl radicals (·OH). In real-world applications of wastewater treatment, however, this term usually refers more specifically to a subset of such chemical processes that employ ozone (O3), hydrogen peroxide (H2O2) and/or UV light. One such type of process is called in situ chemical oxidation.
Photocatalytic water splitting is an artificial photosynthesis process with photocatalysis in a photoelectrochemical cell used for the dissociation of water (H2O) into hydrogen (H
2) and oxygen (O
2), using light. Theoretically, only light energy (photons), water, and a catalyst are needed. This topic is the focus of much research, but thus far no technology has been commercialized.

The blue bottle experiment is a color-changing redox chemical reaction. An aqueous solution containing glucose, sodium hydroxide, methylene blue is prepared in a closed bottle containing some air. Upon standing, it spontaneously turns from blue to colorless due to reduction of methylene blue by the alkaline glucose solution. However, shaking the bottle oxidizes methylene blue back into its blue form. With further shaking, this color-change cycle can be repeated many times. This experiment is a classic chemistry demonstration that can be used in laboratory courses as a general chemistry experiment to study chemical kinetics and reaction mechanism. The reaction also works with other reducing agents besides glucose and other redox indicator dyes besides methylene blue.

Calliandra haematocephala is a species of flowering plants of the genus Calliandra in the family Fabaceae.
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:

Graphitic carbon nitride (g-C3N4) is a family of carbon nitride compounds with a general formula near to C3N4 (albeit typically with non-zero amounts of hydrogen) and two major substructures based on heptazine and poly(triazine imide) units which, depending on reaction conditions, exhibit different degrees of condensation, properties and reactivities.
A photocatalyst activity indicator ink (Paii) is a substance used to identify the presence of an underlying heterogeneous photocatalyst and to measure its activity. Such inks render visible the activity of photocatalytic coatings applied to various "self-cleaning" products. The inks contain a dyestuff that reacts to ultraviolet radiation in the presence of the photocatalytic agent in the coating. They are applied to the coated product and show a color change or disappearance when exposed to ultraviolet radiation. The use of a Paii based on the dye resazurin forms the basis of an ISO standard test for photocatalytic activity.

Cyclohexene oxide is a cycloaliphatic epoxide. It can react in cationic polymerization to poly(cyclohexene oxide). As cyclohexene is monovalent, poly(cyclohexene oxide) is a thermoplastic.
Steven L. Suib is an American inorganic chemist, academic and researcher. He is a Board of Trustees Distinguished Professor of Chemistry at University of Connecticut. He is a director of the Institute of Materials Science and of the Center for Advanced Microscopy and Materials Analysis.












