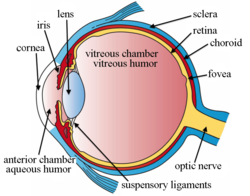
Intravitreal implants are micro device-like inserts injected into the posterior segment of the eye to treat retinal diseases releasing therapeutic drugs at a set rate over a desired period of time. [1] [2] The posterior segment of the eye consists of the sclera, choroid, fovea, vitreous humor, optic nerve, and retina. [3] [4]