
Malonyl-CoA is a coenzyme A derivative of malonic acid.
Polyketide synthases (PKSs) are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages. The biosyntheses of polyketides share striking similarities with fatty acid biosynthesis.
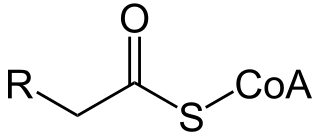
Acyl-CoA is a group of coenzymes that metabolize fatty acids. Acyl-CoA's are susceptible to beta oxidation, forming, ultimately, acetyl-CoA. The acetyl-CoA enters the citric acid cycle, eventually forming several equivalents of ATP. In this way, fats are converted to ATP, the universal biochemical energy carrier.

The long chain fatty acyl-CoA ligase is an enzyme of the ligase family that activates the oxidation of complex fatty acids. Long chain fatty acyl-CoA synthetase catalyzes the formation of fatty acyl-CoA by a two-step process proceeding through an adenylated intermediate. The enzyme catalyzes the following reaction,

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
Diglyceride acyltransferase, DGAT, catalyzes the formation of triglycerides (triacylglycerols) from diacylglycerol and acyl-CoA. The reaction catalyzed by DGAT is considered the terminal and only committed step in the acyl-CoA depedent triglyceride synthesis, univerally important in animal, plants, and microorganisms. The conversion is essential for intestinal absorption and adipose tissue formation in mamalian. DGAT1 are homologous to other membrane-bound O-acyltransferases, but other DGATs are not.
In enzymology, a [acyl-carrier-protein] S-acetyltransferase is an enzyme that catalyzes the reversible chemical reaction
In enzymology, a [acyl-carrier-protein] S-malonyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a beta-ketoacyl-acyl-carrier-protein synthase II (EC 2.3.1.179) is an enzyme that catalyzes the chemical reaction
In enzymology, an erythronolide synthase is an enzyme that catalyzes the chemical reaction
In enzymology, sphingosine N-acyltransferases (ceramide synthases (CerS), EC 2.3.1.24) are enzymes that catalyze the chemical reaction of synthesis of ceramide:
Sterol O-acyltransferase is an intracellular protein located in the endoplasmic reticulum that forms cholesteryl esters from cholesterol.

Sterol O-acyltransferase 2, also known as SOAT2, is an enzyme that in humans is encoded by the SOAT2 gene.

Fatty acyl-CoA reductase 1 is an enzyme that in humans is encoded by the FAR1 gene.

2-acyl-sn-glycero-3-phosphocholines are a class of phospholipids that are intermediates in the metabolism of lipids. Because they result from the hydrolysis of an acyl group from the sn-1 position of phosphatidylcholine, they are also called 1-lysophosphatidylcholine. The synthesis of phosphatidylcholines with specific fatty acids occurs through the synthesis of 1-lysoPC. The formation of various other lipids generates 1-lysoPC as a by-product.
Very-long-chain 3-oxoacyl-CoA synthase (EC 2.3.1.199, very-long-chain 3-ketoacyl-CoA synthase, very-long-chain beta-ketoacyl-CoA synthase, condensing enzyme, CUT1 (gene), CERS6 (gene), FAE1 (gene), KCS (gene), ELO (gene)) is an enzyme with systematic name malonyl-CoA:very-long-chain acyl-CoA malonyltransferase (decarboxylating and thioester-hydrolysing). This enzyme catalyses the following chemical reaction

Ketoacyl synthases (KSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. This reaction is a key step in the fatty acid synthesis cycle, as the resulting acyl chain is two carbon atoms longer than before. KSs exist as individual enzymes, as they do in type II fatty acid synthesis and type II polyketide synthesis, or as domains in large multidomain enzymes, such as type I fatty acid synthases (FASs) and polyketide synthases (PKSs). KSs are divided into five families: KS1, KS2, KS3, KS4, and KS5.

Diacylglycerol O-acyltransferase 1 is an enzyme that in humans is encoded by the DGAT1 gene.

Diacylglycerol O-acyltransferase 2 (DGAT2) is a protein that in humans is encoded by the DGAT2 gene.
An oleaginous microorganism is a type of microbe that accumulates lipid as a normal part of its metabolism. Oleaginous microbes may accumulate an array of different lipid compounds, including polyhydroxyalkanoates, triacylglycerols, and wax esters. Various microorganisms, including bacteria, fungi, and yeast, are known to accumulate lipids. These organisms are often researched for their potential use in producing fuels from waste products.










