
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.

The mineral olivine is a magnesium iron silicate with the chemical formula (Mg,Fe)2SiO4. It is a type of nesosilicate or orthosilicate. The primary component of the Earth's upper mantle, it is a common mineral in Earth's subsurface, but weathers quickly on the surface. For this reason, olivine has been proposed as a good candidate for accelerated weathering to sequester carbon dioxide from the Earth's oceans and atmosphere, as part of climate change mitigation. Olivine also has many other historical uses, such as the gemstone peridot, as well as industrial applications like metalworking processes.

Armalcolite is a titanium-rich mineral with the chemical formula (Mg,Fe2+)Ti2O5. It was first found at Tranquility Base on the Moon in 1969 during the Apollo 11 mission, and is named for Armstrong, Aldrin and Collins, the three Apollo 11 astronauts. Together with tranquillityite and pyroxferroite, it is one of three new minerals that were discovered on the Moon. Armalcolite was later identified at various locations on Earth and has been synthesized in the laboratory. (Tranquillityite and pyroxferroite were also later found at various locations on Earth). The synthesis requires low pressures, high temperatures and rapid quenching from about 1,000 °C to the ambient temperature. Armalcolite breaks down to a mixture of magnesium-rich ilmenite and rutile at temperatures below 1,000 °C, but the conversion slows down with cooling. Because of this quenching requirement, armalcolite is relatively rare and is usually found in association with ilmenite and rutile, among other minerals.

A xenolith is a rock fragment that becomes enveloped in a larger rock during the latter's development and solidification. In geology, the term xenolith is almost exclusively used to describe inclusions in igneous rock entrained during magma ascent, emplacement and eruption. Xenoliths may be engulfed along the margins of a magma chamber, torn loose from the walls of an erupting lava conduit or explosive diatreme or picked up along the base of a flowing body of lava on the Earth's surface. A xenocryst is an individual foreign crystal included within an igneous body. Examples of xenocrysts are quartz crystals in a silica-deficient lava and diamonds within kimberlite diatremes. Xenoliths can be non-uniform within individual locations, even in areas which are spatially limited, e.g. rhyolite-dominated lava of Niijima volcano (Japan) contains two types of gabbroic xenoliths which are of different origin - they were formed in different temperature and pressure conditions.

Coesite is a form (polymorph) of silicon dioxide (SiO2) that is formed when very high pressure (2–3 gigapascals), and moderately high temperature (700 °C, 1,300 °F), are applied to quartz. Coesite was first synthesized by Loring Coes, Jr., a chemist at the Norton Company, in 1953.

Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich end-member of the olivine solid solution series. It is isomorphous with the iron-rich end-member, fayalite. Forsterite crystallizes in the orthorhombic system (space group Pbnm) with cell parameters a 4.75 Å (0.475 nm), b 10.20 Å (1.020 nm) and c 5.98 Å (0.598 nm).

Eclogite is a metamorphic rock containing garnet (almandine-pyrope) hosted in a matrix of sodium-rich pyroxene (omphacite). Accessory minerals include kyanite, rutile, quartz, lawsonite, coesite, amphibole, phengite, paragonite, zoisite, dolomite, corundum and, rarely, diamond. The chemistry of primary and accessory minerals is used to classify three types of eclogite. The broad range of eclogitic compositions has led to a longstanding debate on the origin of eclogite xenoliths as subducted, altered oceanic crust.

Enstatite is a mineral; the magnesium endmember of the pyroxene silicate mineral series enstatite (MgSiO3) – ferrosilite (FeSiO3). The magnesium rich members of the solid solution series are common rock-forming minerals found in igneous and metamorphic rocks. The intermediate composition, (Mg,Fe)SiO
3, has historically been known as hypersthene, although this name has been formally abandoned and replaced by orthopyroxene. When determined petrographically or chemically the composition is given as relative proportions of enstatite (En) and ferrosilite (Fs) (e.g., En80Fs20).

The chlorites are the group of phyllosilicate minerals common in low-grade metamorphic rocks and in altered igneous rocks. Greenschist, formed by metamorphism of basalt or other low-silica volcanic rock, typically contains significant amounts of chlorite.
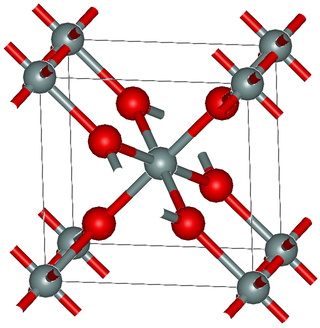
Stishovite is an extremely hard, dense tetragonal form (polymorph) of silicon dioxide. It is very rare on the Earth's surface; however, it may be a predominant form of silicon dioxide in the Earth, especially in the lower mantle.

Clinohumite is an uncommon member of the humite group, a magnesium silicate according to the chemical formula (Mg, Fe)9(SiO4)4(F,OH)2. The formula can be thought of as four olivine (Mg2SiO4), plus one brucite (Mg(OH)2). Indeed, the mineral is essentially a hydrated olivine and occurs in altered ultramafic rocks and carbonatites. Most commonly found as tiny indistinct grains, large euhedral clinohumite crystals are sought by collectors and occasionally fashioned into bright, yellow-orange gemstones. Only two sources of gem-quality material are known: the Pamir Mountains of Tajikistan, and the Taymyr region of northern Siberia. It is one of two humite group minerals that have been cut into gems, the other being the much more common chondrodite.

Chondrodite is a nesosilicate mineral with formula (Mg,Fe)
5(SiO
4)
2(F,OH,O)
2. Although it is a fairly rare mineral, it is the most frequently encountered member of the humite group of minerals. It is formed in hydrothermal deposits from locally metamorphosed dolomite. It is also found associated with skarn and serpentinite. It was discovered in 1817 at Pargas in Finland, and named from the Greek for "granule", which is a common habit for this mineral.

Pyroxferroite (Fe2+,Ca)SiO3 is a single chain inosilicate. It is mostly composed of iron, silicon and oxygen, with smaller fractions of calcium and several other metals. Together with armalcolite and tranquillityite, it is one of the three minerals which were discovered on the Moon during the 1969 Apollo 11 mission. It was then found in Lunar and Martian meteorites as well as a mineral in the Earth's crust. Pyroxferroite can also be produced by annealing synthetic clinopyroxene at high pressures and temperatures. The mineral is metastable and gradually decomposes at ambient conditions, but this process can take billions of years.

Omphacite is a member of the clinopyroxene group of silicate minerals with formula: (Ca, Na)(Mg, Fe2+, Al)Si2O6. It is a variably deep to pale green or nearly colorless variety of clinopyroxene. It normally appears in eclogite, which is the high-pressure metamorphic rock of basalt. Omphacite is the solid solution of Fe-bearing diopside and jadeite. It crystallizes in the monoclinic system with prismatic, typically twinned forms, though usually anhedral. Its space group can be P2/n or C2/c depending on the thermal history. It exhibits the typical near 90° pyroxene cleavage. It is brittle with specific gravity of 3.29 to 3.39 and a Mohs hardness of 5 to 6.

Lawsonite is a hydrous calcium aluminium sorosilicate mineral with formula CaAl2Si2O7(OH)2·H2O. Lawsonite crystallizes in the orthorhombic system in prismatic, often tabular crystals. Crystal twinning is common. It forms transparent to translucent colorless, white, pink, and bluish to pinkish grey glassy to greasy crystals. Refractive indices are nα = 1.665, nβ = 1.672 – 1.676, and nγ = 1.684 – 1.686. It is typically almost colorless in thin section, but some lawsonite is pleochroic from colorless to pale yellow to pale blue, depending on orientation. The mineral has a Mohs hardness of 7.5 and a specific gravity of 3.09. It has perfect cleavage in two directions and a brittle fracture. Not to be confused with Larsonite, a fossiliferous jasper mined in Nevada.

Wadsleyite is an orthorhombic mineral with the formula β-(Mg,Fe)2SiO4. It was first found in nature in the Peace River meteorite from Alberta, Canada. It is formed by a phase transformation from olivine (α-(Mg,Fe)2SiO4) under increasing pressure and eventually transforms into spinel-structured ringwoodite (γ-(Mg,Fe)2SiO4) as pressure increases further. The structure can take up a limited amount of other bivalent cations instead of magnesium, but contrary to the α and γ structures, a β structure with the sum formula Fe2SiO4 is not thermodynamically stable. Its cell parameters are approximately a = 5.7 Å, b = 11.71 Å and c = 8.24 Å.

Ringwoodite is a high-pressure phase of Mg2SiO4 (magnesium silicate) formed at high temperatures and pressures of the Earth's mantle between 525 and 660 km (326 and 410 mi) depth. It may also contain iron and hydrogen. It is polymorphous with the olivine phase forsterite (a magnesium iron silicate).
Akimotoite is a rare silicate mineral in the ilmenite group of minerals, with the chemical formula (Mg,Fe)SiO3. It is polymorphous with pyroxene and with bridgmanite, a natural silicate perovskite that is the most abundant mineral in Earth's silicate mantle. Akimotoite has a vitreous luster, is colorless, and has a white or colorless streak. It crystallizes in the trigonal crystal system in space group R3. It is the silicon analogue of geikielite (MgTiO3).

Pimelite was discredited as a mineral species by the International Mineralogical Association (IMA) in 2006, in an article which suggests that "pimelite" specimens are probably willemseite, or kerolite. This was a mass discreditation, and not based on any re-examination of the type material. Nevertheless, a considerable number of papers have been written, verifying that pimelite is a nickel-dominant smectite. It is always possible to redefine a mineral wrongly discredited.
Silicate perovskite is either (Mg,Fe)SiO3 or CaSiO3 when arranged in a perovskite structure. Silicate perovskites are not stable at Earth's surface, and mainly exist in the lower part of Earth's mantle, between about 670 and 2,700 km depth. They are thought to form the main mineral phases, together with ferropericlase.

















