Ferromagnetism is a property of certain materials that results in a significant, observable magnetic permeability, and in many cases, a significant magnetic coercivity, allowing the material to form a permanent magnet. Ferromagnetic materials are familiar metals that are noticeably attracted to a magnet, a consequence of their substantial magnetic permeability. Magnetic permeability describes the induced magnetization of a material due to the presence of an external magnetic field. This temporarily induced magnetization, for example, inside a steel plate, accounts for its attraction to the permanent magnet. Whether or not that steel plate acquires a permanent magnetization itself depends not only on the strength of the applied field but on the so-called coercivity of the ferromagnetic material, which can vary greatly.

In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter.

In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism. The phenomenon of antiferromagnetism was first introduced by Lev Landau in 1933.

In physics and materials science, the Curie temperature (TC), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Curie temperature is named after Pierre Curie, who showed that magnetism was lost at a critical temperature.

Heusler compounds are magnetic intermetallics with face-centered cubic crystal structure and a composition of XYZ (half-Heuslers) or X2YZ (full-Heuslers), where X and Y are transition metals and Z is in the p-block. The term derives from the name of German mining engineer and chemist Friedrich Heusler, who studied such a compound (Cu2MnAl) in 1903. Many of these compounds exhibit properties relevant to spintronics, such as magnetoresistance, variations of the Hall effect, ferro-, antiferro-, and ferrimagnetism, half- and semimetallicity, semiconductivity with spin filter ability, superconductivity, topological band structure and are actively studied as Thermoelectric materials. Their magnetism results from a double-exchange mechanism between neighboring magnetic ions. Manganese, which sits at the body centers of the cubic structure, was the magnetic ion in the first Heusler compound discovered. (See the Bethe–Slater curve for details of why this happens.)

Helimagnetism is a form of magnetic ordering where spins of neighbouring magnetic moments arrange themselves in a spiral or helical pattern, with a characteristic turn angle of somewhere between 0 and 180 degrees. It results from the competition between ferromagnetic and antiferromagnetic exchange interactions. It is possible to view ferromagnetism and antiferromagnetism as helimagnetic structures with characteristic turn angles of 0 and 180 degrees respectively. Helimagnetic order breaks spatial inversion symmetry, as it can be either left-handed or right-handed in nature.

Lanthanum strontium manganite (LSM or LSMO) is an oxide ceramic material with the general formula La1−xSrxMnO3, where x describes the doping level.
Gallium manganese arsenide, chemical formula (Ga,Mn)As is a magnetic semiconductor. It is based on the world's second most commonly used semiconductor, gallium arsenide,, and readily compatible with existing semiconductor technologies. Differently from other dilute magnetic semiconductors, such as the majority of those based on II-VI semiconductors, it is not paramagnetic but ferromagnetic, and hence exhibits hysteretic magnetization behavior. This memory effect is of importance for the creation of persistent devices. In (Ga,Mn)As, the manganese atoms provide a magnetic moment, and each also acts as an acceptor, making it a p-type material. The presence of carriers allows the material to be used for spin-polarized currents. In contrast, many other ferromagnetic magnetic semiconductors are strongly insulating and so do not possess free carriers. (Ga,Mn)As is therefore a candidate as a spintronic material.
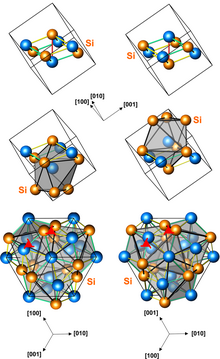
The term magnetic structure of a material pertains to the ordered arrangement of magnetic spins, typically within an ordered crystallographic lattice. Its study is a branch of solid-state physics.

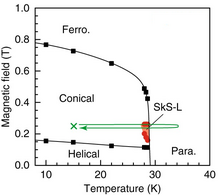
In physics, magnetic skyrmions are statically stable soliton which have been predicted theoretically and observed experimentally in condensed matter systems. Magnetic skyrmions can be formed in magnetic materials in their 'bulk' such as in manganese monosilicide (MnSi), or in magnetic thin films. They can be achiral, or chiral in nature, and may exist both as dynamic excitations or stable or metastable states. Although the broad lines defining magnetic skyrmions have been established de facto, there exist a variety of interpretations with subtle differences.

Iron monosilicide (FeSi) is an intermetallic compound, a silicide of iron that occurs in nature as the rare mineral naquite. It is a narrow-bandgap semiconductor with a room-temperature electrical resistivity of around 10 kΩ·cm and unusual magnetic properties at low temperatures. FeSi has a cubic crystal lattice with no inversion center; therefore its magnetic structure is helical, with right-hand and left-handed chiralities.

Iron germanide (FeGe) is an intermetallic compound, a germanide of iron. At ambient conditions it crystallizes in three polymorphs with monoclinic, hexagonal and cubic structures. The cubic polymorph has no inversion center, it is therefore helical, with right-hand and left-handed chiralities.

Manganese germanide (MnGe) is an intermetallic compound, a germanide of manganese. Its crystals have a cubic symmetry with no inversion center, they are therefore helical, with right-hand and left-handed chiralities.

Cobalt germanide (CoGe) is an intermetallic compound, a germanide of cobalt.

Cobalt monosilicide (CoSi) is an intermetallic compound, a silicide of cobalt. It is a diamagnetic semimetal with an electrical resistivity of around 1 mΩ·cm.

Manganese disilicide (MnSi2) is an intermetallic compound, a silicide of manganese. It is a non-stoichiometric compound, with a silicon deficiency expressed as MnSi2–x. Crystal structures of many MnSi2–x compounds resemble a chimney ladder and are called Nowotny phases. They include MnSi2 (x=0), Mn4Si7 (x=0.250), Mn11Si19 (x=0.273), Mn15Si26 (x=0.267) and Mn27Si47 (x=0.259). These phases have very similar unit cells whose length varies from 1.75 nm for MnSi2 or Mn4Si7, which have almost the same structures, to 11.8 nm for Mn27Si47.
Copper oxide selenite is an inorganic compound with the chemical formula Cu2OSeO3. It is an electrically insulating, piezoelectric and piezomagnetic material, which becomes a ferrimagnet upon cooling below 58 K. As of 2021, Cu2OSeO3 is the only insulating material that hosts magnetic skyrmions.
Chromium(IV) silicide or chromium monosilicide is an inorganic compound of chromium and silicon with a chemical formula of CrSi. It is a metal with an electrical resistivity of ca. 2×10−4 Ω·cm.

Manganese arsenide (MnAs) is an intermetallic compound, an arsenide of manganese. It forms ferromagnetic crystals with hexagonal (NiAs-type) crystal structure, which convert to the paramagnetic orthorhombic β-phase upon heating to 45 °C (113 °F). MnAs has potential applications in spintronics, for electrical spin injection into GaAs and Si based devices.
Mavlyanovite is a manganese-silicon mineral with formula Mn5Si3. It was named after Gani Mavlyanov, an Uzbek geologist who lived from 1910 to 1988.