
In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

Heusler compounds are magnetic intermetallics with face-centered cubic crystal structure and a composition of XYZ (half-Heuslers) or X2YZ (full-Heuslers), where X and Y are transition metals and Z is in the p-block. The term derives from the name of German mining engineer and chemist Friedrich Heusler, who studied such a compound (Cu2MnAl) in 1903. Many of these compounds exhibit properties relevant to spintronics, such as magnetoresistance, variations of the Hall effect, ferro-, antiferro-, and ferrimagnetism, half- and semimetallicity, semiconductivity with spin filter ability, superconductivity, topological band structure and are actively studied as Thermoelectric materials. Their magnetism results from a double-exchange mechanism between neighboring magnetic ions. Manganese, which sits at the body centers of the cubic structure, was the magnetic ion in the first Heusler compound discovered. (See the Bethe–Slater curve for details of why this happens.)
Plutonium hydride is a non-stoichiometric chemical compound with the formula PuH2+x. It is one of two characterised hydrides of plutonium, the other is PuH3. PuH2 is non-stoichiometric with a composition range of PuH2 – PuH2.7. Additionally metastable stoichiometries with an excess of hydrogen (PuH2.7 – PuH3) can be formed. PuH2 has a cubic structure. It is readily formed from the elements at 1 atmosphere at 100–200 °C: When the stoichiometry is close to PuH2 it has a silver appearance, but gets blacker as the hydrogen content increases, additionally the color change is associated with a reduction in conductivity.
Complex metallic alloys (CMAs) or complex intermetallics (CIMs) are intermetallic compounds characterized by the following structural features:
- large unit cells, comprising some tens up to thousands of atoms,
- the presence of well-defined atom clusters, frequently of icosahedral point group symmetry,
- the occurrence of inherent disorder in the ideal structure.
Nickel aluminide typically refers to the one of the two most widely used compounds, Ni3Al or NiAl, however is generally any aluminide from the Ni-Al system. These alloys are widely used due to their corrosion resistance, low-density and easy production. Ni3Al is of specific interest as the strengthening γ' phase precipitate in nickel-based superalloys allowing for high temperature strength up to 0.7-0.8 of its melting temperature. Meanwhile, NiAl displays excellent properties such as low-density (lower than that of Ni3Al), good thermal conductivity, oxidation resistance and high melting temperature. These properties, make it ideal for special high temperature applications like coatings on blades in gas turbines and jet engines. However, both these alloys do have the disadvantage of being quite brittle at room temperature while Ni3Al remains brittle at high temperatures as well. Although, it has been shown that Ni3Al can be made ductile when manufactured as a single crystal as opposed to polycrystalline. Another application was demonstrated in 2005, when the most abrasion-resistant material was reportedly created by embedding diamonds in a matrix of nickel aluminide.

Magnesium hydride is the chemical compound with the molecular formula MgH2. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.

Binary compounds of silicon are binary chemical compounds containing silicon and one other chemical element. Technically the term silicide is reserved for any compounds containing silicon bonded to a more electropositive element. Binary silicon compounds can be grouped into several classes. Saltlike silicides are formed with the electropositive s-block metals. Covalent silicides and silicon compounds occur with hydrogen and the elements in groups 10 to 17.
Bismuth selenide is a gray compound of bismuth and selenium also known as bismuth(III) selenide. It is a semiconductor and a thermoelectric material. In its pure state it has a topological insulator ground-state. While perfect stoichiometric bismuth selenide should be a semiconductor naturally occurring selenium vacancies act as electron donors and it often acts as a semimetal in its as grown phase. Topologically protected Dirac cone surface states have been observed in Bismuth selenide and its insulating derivatives leading to intrinsic topological insulators, which later became the subject of world-wide scientific research.
Chromium hydrides are compounds of chromium and hydrogen, and possibly other elements. Intermetallic compounds with not-quite-stoichometric quantities of hydrogen exist, as well as highly reactive molecules. When present at low concentrations, hydrogen and certain other elements alloyed with chromium act as softening agents that enables the movement of dislocations that otherwise not occur in the crystal lattices of chromium atoms.
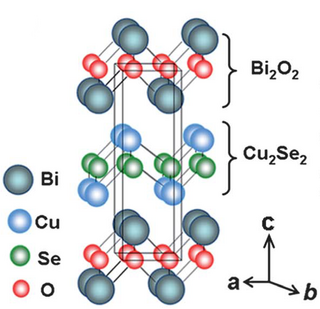
Oxyselenides are a group of chemical compounds that contain oxygen and selenium atoms. Oxyselenides can form a wide range of structures in compounds containing various transition metals, and thus can exhibit a wide range of properties. Most importantly, oxyselenides have a wide range of thermal conductivity, which can be controlled with changes in temperature in order to adjust their thermoelectric performance. Current research on oxyselenides indicates their potential for significant application in electronic materials.
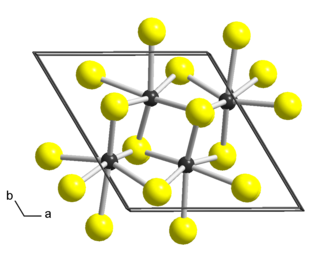
Rhenium disulfide is an inorganic compound of rhenium and sulfur with the formula ReS2. It has a layered structure where atoms are strongly bonded within each layer. The layers are held together by weak Van der Waals bonds, and can be easily peeled off from the bulk material.

Yttrium(III) nitrate is an inorganic compound, a salt with the formula Y(NO3)3. The hexahydrate is the most common form commercially available.
Yukiko Ogawa is a materials science researcher at the National Institute for Materials Science in Tsukuba, Ibaraki Prefecture, Japan. Ogawa’s research focuses on next-generation structural materials — particularly lightweight ones such as magnesium alloys — that show promising potential to improve fuel efficiency in vehicles, make electronic devices more portable and open up new possibilities in medical devices. The use of magnesium alloys has been limited as they are difficult to shape into new forms. But Ogawa succeeded in controlling the microstructure and mechanical properties of magnesium by heat treatment, which had previously been considered impossible. For her efforts in creating the next generation of smart materials, Ogawa was recognized as a 2018 L’Oréal-UNESCO For Women in Science International Rising Talent. Ogawa grew up in Komaki, Aichi Prefecture and studied engineering at Tohoku University in Sendai.
Carbohydrides are solid compounds in one phase composed of a metal with carbon and hydrogen in the form of carbide and hydride ions. The term carbohydride can also refer to a hydrocarbon.

Dysprosium(III) hydroxide is an inorganic compound with the chemical formula Dy(OH)3.
Borate sulfides are chemical mixed anion compounds that contain any kind of borate and sulfide ions. They are distinct from thioborates in which sulfur atoms replace oxygen in borates. There are also analogous borate selenides, with selenium ions instead of sulfur.
Cerium(III) sulfide, also known as cerium sesquisulfide, is an inorganic compound with the formula Ce2S3. It is the sulfide salt of cerium(III) and exists as three polymorphs with different crystal structures.
Phosphide carbides or carbide phosphides are compounds containing anions composed of carbide (C4−) and phosphide (P3−). They can be considered as mixed anion compounds. Related compounds include the phosphide silicides, germanide phosphides, arsenide carbides, nitride carbides and silicide carbides.
Silicide carbides or carbide silicides are compounds containing anions composed of silicide (Si4−) and carbide (C4−) or clusters therof. They can be considered as mixed anion compounds or intermetallic compounds, as silicon could be considered as a semimetal.
Tellurogallates are chemical compounds which contain anionic units of tellurium connected to gallium. They can be considered as gallates where tellurium substitutes for oxygen. Similar compounds include the thiogallates, selenogallates, telluroaluminates, telluroindates and thiostannates. They are in the category of chalcogenotrielates or more broadly tellurometallates or chalcogenometallates.







