
Mefloquine, sold under the brand name Lariam among others, is a medication used to prevent or treat malaria. When used for prevention it is typically started before potential exposure and continued for several weeks after potential exposure. It can be used to treat mild or moderate malaria but is not recommended for severe malaria. It is taken by mouth.
Antimalarial medications or simply antimalarials are a type of antiparasitic chemical agent, often naturally derived, that can be used to treat or to prevent malaria, in the latter case, most often aiming at two susceptible target groups, young children and pregnant women. As of 2018, modern treatments, including for severe malaria, continued to depend on therapies deriving historically from quinine and artesunate, both parenteral (injectable) drugs, expanding from there into the many classes of available modern drugs. Incidence and distribution of the disease is expected to remain high, globally, for many years to come; moreover, known antimalarial drugs have repeatedly been observed to elicit resistance in the malaria parasite—including for combination therapies featuring artemisinin, a drug of last resort, where resistance has now been observed in Southeast Asia. As such, the needs for new antimalarial agents and new strategies of treatment remain important priorities in tropical medicine. As well, despite very positive outcomes from many modern treatments, serious side effects can impact some individuals taking standard doses.
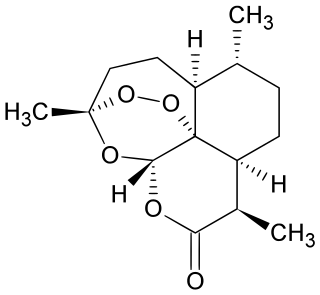
Artemisinin and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery. Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium. Artemisinin is extracted from the plant Artemisia annua a herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.

Artemether is a medication used for the treatment of malaria. The injectable form is specifically used for severe malaria rather than quinine. In adults, it may not be as effective as artesunate. It is given by injection in a muscle. It is also available by mouth in combination with lumefantrine, known as artemether/lumefantrine.

Artesunate (AS) is a medication used to treat malaria. The intravenous form is preferred to quinine for severe malaria. Often it is used as part of combination therapy, such as artesunate plus mefloquine. It is not used for the prevention of malaria. Artesunate can be given by injection into a vein, injection into a muscle, by mouth, and by rectum.

Primaquine is a medication used to treat and prevent malaria and to treat Pneumocystis pneumonia. Specifically it is used for malaria due to Plasmodium vivax and Plasmodium ovale along with other medications and for prevention if other options cannot be used. It is an alternative treatment for Pneumocystis pneumonia together with clindamycin. It is taken by mouth.

Proguanil, also known as chlorguanide and chloroguanide, is a medication used to treat and prevent malaria. It is often used together with chloroquine or atovaquone. When used with chloroquine the combination will treat mild chloroquine resistant malaria. It is taken by mouth.
Artemether/lumefantrine, sold under the trade name Coartem among others, is a combination of the two medications artemether and lumefantrine. It is used to treat malaria caused by Plasmodium falciparum that is not treatable with chloroquine. It is not typically used to prevent malaria. It is taken by mouth.

The Drugs for Neglected Diseases initiative (DNDi) is a collaborative, patients' needs-driven, non-profit drug research and development (R&D) organization that is developing new treatments for neglected diseases, notably leishmaniasis, sleeping sickness, Chagas disease, malaria, filarial diseases, mycetoma, paediatric HIV, cryptococcal meningitis, hepatitis C, and dengue. DNDi's malaria activities were transferred to Medicines for Malaria Venture (MMV) in 2015.

Tafenoquine, sold under the brand name Krintafel among others, is a medication used to prevent and to treat malaria. With respect to acute malaria, it is used together with other medications to prevent relapse by Plasmodium vivax. It may be used to prevent all types of malaria. It is taken by mouth.

Unitaid is a global health initiative that works with partners to bring about innovations to prevent, diagnose and treat major diseases in low- and middle-income countries, with an emphasis on tuberculosis, malaria, and HIV/AIDS and its deadly co-infections. Founded in 2006, the organization funds the final stages of research and development of new drugs, diagnostics and disease-prevention tools, helps produce data supporting guidelines for their use, and works to allow more affordable generic medicines to enter the marketplace in low- and middle-income countries. Hosted by the World Health Organization (WHO) in Geneva, Unitaid was established by the governments of Brazil, Chile, France, Norway and the United Kingdom.
Artesunate/amodiaquine, sold under the trade name Camoquin among others, is a medication used for the treatment of malaria. It is a fixed-dose combination of artesunate and amodiaquine. Specifically it recommended for acute uncomplicated Plasmodium falciparum malaria. It is taken by mouth.
Intermittent preventive therapy or intermittent preventive treatment (IPT) is a public health intervention aimed at treating and preventing malaria episodes in infants (IPTi), children (IPTc), schoolchildren (IPTsc) and pregnant women (IPTp). The intervention builds on two tested malaria control strategies to clear existing parasites and to prevent new infections (prophylaxis).

Arterolane, also known as OZ277 or RBx 11160, is a substance that was tested for antimalarial activity by Ranbaxy Laboratories. It was discovered by US and European scientists who were coordinated by the Medicines for Malaria Venture (MMV). Its molecular structure is uncommon for pharmacological compounds in that it has both an ozonide (trioxolane) group and an adamantane substituent.

Cipargamin is an experimental synthetic antimalarial drug belonging to the spiroindolone class. The compound was developed at the Novartis Institute for Tropical Diseases in Singapore, through a collaboration with the Genomics Institute of the Novartis Research Foundation (GNF), the Biomedical Primate Research Centre and the Swiss Tropical Institute.
Pregnancy-associated malaria (PAM) or placental malaria is a presentation of the common illness that is particularly life-threatening to both mother and developing fetus. PAM is caused primarily by infection with Plasmodium falciparum, the most dangerous of the four species of malaria-causing parasites that infect humans. During pregnancy, a woman faces a much higher risk of contracting malaria and of associated complications. Prevention and treatment of malaria are essential components of prenatal care in areas where the parasite is endemic – tropical and subtropical geographic areas. Placental malaria has also been demonstrated to occur in animal models, including in rodent and non-human primate models.

Ganaplacide is a drug in development by Novartis for the purpose of treating malaria. It belongs to the class of the imidazolopiperazines. It has shown activity against the Plasmodium falciparum and Plasmodium vivax forms of the malaria parasite.

Elizabeth Ann Winzeler is an American microbiologist and geneticist. She is a professor in the Division of Host-Microbe Systems and Therapeutics of the School of Medicine at the University of California at San Diego. Although she works in a variety of different disease areas, most research focuses on developing better medicines for the treatment and eradication of malaria.

Matthew Houghton Todd is a British chemist and the Professor and Chair of Drug Discovery of the School of Pharmacy at University College London. He is the founder of Open Source Malaria (OSM) and his research focuses on drug discovery and development for this disease. Recently, he has expanded to other areas, particularly neglected diseases such as tuberculosis and mycetoma in the Open Source Tuberculosis (OSTB) and Open Source Mycetoma (MycetOS) project, through a collaboration with the Drugs for Neglected Diseases Initiative and Erasmus MC. In addition, he has some research activity in catalysis and methodology.

David A. Fidock, is the CS Hamish Young Professor of Microbiology and Immunology and Professor of Medical Sciences at Columbia University Irving Medical Center in Manhattan.














