Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones.

Miconazole, sold under the brand name Monistat among others, is an antifungal medication used to treat ring worm, pityriasis versicolor, and yeast infections of the skin or vagina. It is used for ring worm of the body, groin, and feet. It is applied to the skin or vagina as a cream or ointment.
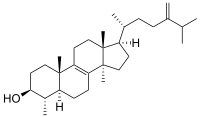
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast plant steroids are produced via cycloartenol.

CYP27A1 is a gene encoding a cytochrome P450 oxidase, and is commonly known as sterol 27-hydroxylase. This enzyme is located in many different tissues where it is found within the mitochondria. It is most prominently involved in the biosynthesis of bile acids.

Cholesterol 7 alpha-hydroxylase also known as cholesterol 7-alpha-monooxygenase or cytochrome P450 7A1 (CYP7A1) is an enzyme that in humans is encoded by the CYP7A1 gene which has an important role in cholesterol metabolism. It is a cytochrome P450 enzyme, which belongs to the oxidoreductase class, and converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid synthesis.

Lanosterol 14α-demethylase (CYP51A1) is a cytochrome P450 enzyme that is involved in the conversion of lanosterol to 4,4-dimethylcholesta-8(9),14,24-trien-3β-ol. The cytochrome P450 isoenzymes are a conserved group of proteins that serve as key players in the metabolism of organic substances and the biosynthesis of important steroids, lipids, and vitamins in eukaryotes. As a member of this family, lanosterol 14α-demethylase is responsible for an essential step in the biosynthesis of sterols. In particular, this protein catalyzes the removal of the C-14α-methyl group from lanosterol. This demethylation step is regarded as the initial checkpoint in the transformation of lanosterol to other sterols that are widely used within the cell.
In enzymology, a cholestanetriol 26-monooxygenase (EC 1.14.13.15) is an enzyme that catalyzes the chemical reaction

In enzymology, a sterol 14-demethylase (EC 1.14.13.70) is an enzyme that catalyzes the chemical reaction

Leukotriene-B(4) omega-hydroxylase 1 is an enzyme protein involved in the metabolism of various endogenous substrates and xenobiotics. The most notable substrate of the enzyme is leukotriene B4, a potent mediator of inflammation. The CYP4F2 gene encodes the enzyme in humans.

4,4′-Dihydroxybenzophenone is an organic compound with the formula (HOC6H4)2CO. This off-white solid is a precursor to or a degradation product of diverse commercial materials. It is a potential endocrine disruptor.

CYP8B1 also known as sterol 12-alpha-hydroxylase is a protein which in humans is encoded by the CYP8B1 gene.
Taurochenodeoxycholate 6alpha-hydroxylase (EC 1.14.13.97, CYP3A4, CYP4A21, taurochenodeoxycholate 6alpha-monooxygenase) is an enzyme with systematic name taurochenodeoxycholate,NADPH:oxygen oxidoreductase (6alpha-hydroxylating). This enzyme catalyses the following chemical reaction
Cholest-4-en-3-one 26-monooxygenase (EC 1.14.13.141, CYP125, CYP125A1, cholest-4-en-3-one 27-monooxygenase) is an enzyme with systematic name cholest-4-en-3-one,NADH:oxygen oxidoreductase (26-hydroxylating). This enzyme catalyses the following chemical reaction

VNI is an experimental drug for treating Chagas disease currently being studied at Vanderbilt University. The molecule acts by inhibiting Trypanosoma cruzi sterol 14α-desmethylase activity in vitro. It exhibits no toxicity in mouse cells and unlike the related compounds posaconazole and fluconazole, increasing the dose is not required to maintain anti-parasitic activity.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.
ERG11 or Sterol 14-demethylase is a fungal cytochrome P450 enzyme originally from Saccharomyces cerevisiae, belongs to family CYP51, with the CYP Symbol CYP51F1. ERG11 catalyzes the C14-demethylation of lanosterol to 4,4'-dimethyl cholesta-8,14,24-triene-3-beta-ol which is the first step of biosynthesis of the zymosterol, zymosterol will be further converted into Ergosterol.
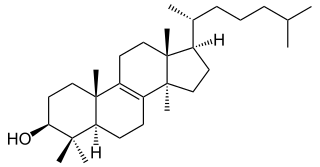
Dihydrolanosterol, or 24,25-Dihydrolanosterol, also called Lanostenol, is a sterol and the C24-25 hydrogenated products of lanosterol, dihydrolanosterol can be demethylated by mammal or yeast cytochrome P450 sterol 14alpha-demethylase.
ERG5 or Sterol 22-desaturase is a cytochrome P450 enzyme in the ergosterol biosynthesis pathway of fungi Saccharomyces cerevisiae, with the CYP Symbol CYP61A1. CYP61A1 is one of only three P450 enzyme found in baker's yeast, the other two are CYP51F1 and CYP56A1. The ortholog in Schizosaccharomyces pombe, was named CYP61A3 for historical reasons, and is only one of two P450 enzyme found with CYP51F1. ERG5 catalyzes the C22-C23 double bond formation on the sterol side chain of ergostatrienol to convert it into ergostatetraenol, then the C24 double bond of ergostatetrenol will be hydrogenation reduced into ergosterol by ERG4.
Cytochrome P450, family 710, also known as CYP710, is a plant cytochrome P450 monooxygenase family, the proteins encoded by its family members are mainly sterol 22-desaturase, which was widely distributed in plants, and take participate in Phytosteroidogenesis. CYP710 family is considered to be the plant orthologous of fungi CYP61 family, which is lost in animal. The CYP61/CYP710 ancestor gene diverged from a gene duplication of ancestor CYP51 in early eukaryotes
Cytochrome P450, family 12, also known as CYP12, is a cytochrome P450 family found in insect genome belongs to Mitochondrial clan CYPs, which is located in the inner membrane of mitochondria(IMM). The first gene identified in this family is the CYP12A1 from the Musca domestica, which is involved in insecticide resistance.