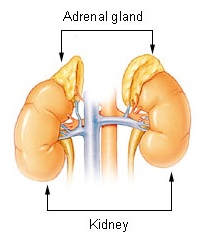
The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.

The endocrine system is a messenger system in an organism comprising feedback loops of hormones that are released by internal glands directly into the circulatory system and that target and regulate distant organs. In vertebrates, the hypothalamus is the neural control center for all endocrine systems.

The salivary glands in many vertebrates including mammals are exocrine glands that produce saliva through a system of ducts. Humans have three paired major salivary glands, as well as hundreds of minor salivary glands. Salivary glands can be classified as serous, mucous, or seromucous (mixed).

Parathyroid glands are small endocrine glands in the neck of humans and other tetrapods. Humans usually have four parathyroid glands, located on the back of the thyroid gland in variable locations. The parathyroid gland produces and secretes parathyroid hormone in response to a low blood calcium, which plays a key role in regulating the amount of calcium in the blood and within the bones.

Leydig cells, also known as interstitial cells of the testes and interstitial cells of Leydig, are found adjacent to the seminiferous tubules in the testicle and produce testosterone in the presence of luteinizing hormone (LH). They are polyhedral in shape and have a large, prominent nucleus, an eosinophilic cytoplasm, and numerous lipid-filled vesicles.

Parathyroid hormone-related protein (PTHrP) is a proteinaceous hormone and a member of the parathyroid hormone family secreted by mesenchymal stem cells. It is occasionally secreted by cancer cells. However, it also has normal functions in bone, teeth, vascular tissues and other tissues.

An osteoclast is a type of bone cell that breaks down bone tissue. This function is critical in the maintenance, repair, and remodeling of bones of the vertebral skeleton. The osteoclast disassembles and digests the composite of hydrated protein and mineral at a molecular level by secreting acid and a collagenase, a process known as bone resorption. This process also helps regulate the level of blood calcium.

Parafollicular cells, also called C cells, are neuroendocrine cells in the thyroid. The primary function of these cells is to secrete calcitonin. They are located adjacent to the thyroid follicles and reside in the connective tissue. These cells are large and have a pale stain compared with the follicular cells. In teleost and avian species these cells occupy a structure outside the thyroid gland named the ultimopharyngeal body.

Parathyroid chief cells are one of the two cell types of the parathyroid glands, along with oxyphil cells. The chief cells are much more prevalent in the parathyroid gland than the oxyphil cells. It is perceived that oxyphil cells may be derived from chief cells at puberty, as they are not present at birth like chief cells.

Sweat glands, also known as sudoriferous or sudoriparous glands, from Latin sudor 'sweat', are small tubular structures of the skin that produce sweat. Sweat glands are a type of exocrine gland, which are glands that produce and secrete substances onto an epithelial surface by way of a duct. There are two main types of sweat glands that differ in their structure, function, secretory product, mechanism of excretion, anatomic distribution, and distribution across species:
Neuroendocrine cells are cells that receive neuronal input and, as a consequence of this input, release messenger molecules (hormones) into the blood. In this way they bring about an integration between the nervous system and the endocrine system, a process known as neuroendocrine integration. An example of a neuroendocrine cell is a cell of the adrenal medulla, which releases adrenaline to the blood. The adrenal medullary cells are controlled by the sympathetic division of the autonomic nervous system. These cells are modified postganglionic neurons. Autonomic nerve fibers lead directly to them from the central nervous system. The adrenal medullary hormones are kept in vesicles much in the same way neurotransmitters are kept in neuronal vesicles. Hormonal effects can last up to ten times longer than those of neurotransmitters. Sympathetic nerve fiber impulses stimulate the release of adrenal medullary hormones. In this way the sympathetic division of the autonomic nervous system and the medullary secretions function together.
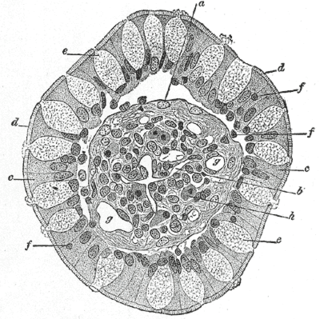
Goblet cells are simple columnar epithelial cells that secrete gel-forming mucins, like mucin 5AC. The goblet cells mainly use the merocrine method of secretion, secreting vesicles into a duct, but may use apocrine methods, budding off their secretions, when under stress. The term goblet refers to the cell's goblet-like shape. The apical portion is shaped like a cup, as it is distended by abundant mucus laden granules; its basal portion lacks these granules and is shaped like a stem.
In human anatomy, there are three types of chief cells, the gastric chief cell, the parathyroid chief cell, and the type 1 chief cells found in the carotid body.

Tertiary hyperparathyroidism is a condition involving the overproduction of the hormone, parathyroid hormone, produced by the parathyroid glands. The parathyroid glands are involved in monitoring and regulating blood calcium levels and respond by either producing or ceasing to produce parathyroid hormone. Anatomically, these glands are located in the neck, para-lateral to the thyroid gland, which does not have any influence in the production of parathyroid hormone. Parathyroid hormone is released by the parathyroid glands in response to low blood calcium circulation. Persistent low levels of circulating calcium are thought to be the catalyst in the progressive development of adenoma, in the parathyroid glands resulting in primary hyperparathyroidism. While primary hyperparathyroidism is the most common form of this condition, secondary and tertiary are thought to result due to chronic kidney disease (CKD). Estimates of CKD prevalence in the global community range from 11 to 13% which translate to a large portion of the global population at risk of developing tertiary hyperparathyroidism. Tertiary hyperparathyroidism was first described in the late 1960s and had been misdiagnosed as primary prior to this. Unlike primary hyperparathyroidism, the tertiary form presents as a progressive stage of resolved secondary hyperparathyroidism with biochemical hallmarks that include elevated calcium ion levels in the blood, hypercalcemia, along with autonomous production of parathyroid hormone and adenoma in all four parathyroid glands. Upon diagnosis treatment of tertiary hyperparathyroidism usually leads to a surgical intervention.

Kisspeptins are proteins encoded by the KISS1 gene in humans. Kisspeptins are ligands of the G-protein coupled receptor, GPR54. Kiss1 was originally identified as a human metastasis suppressor gene that has the ability to suppress melanoma and breast cancer metastasis. Kisspeptin-GPR54 signaling has an important role in initiating secretion of gonadotropin-releasing hormone (GnRH) at puberty, the extent of which is an area of ongoing research. Gonadotropin-releasing hormone is released from the hypothalamus to act on the anterior pituitary triggering the release of luteinizing hormone (LH), and follicle stimulating hormone (FSH). These gonadotropic hormones lead to sexual maturation and gametogenesis. Disrupting GPR54 signaling can cause hypogonadotrophic hypogonadism in rodents and humans. The Kiss1 gene is located on chromosome 1. It is transcribed in the brain, adrenal gland, and pancreas.

Estrogen receptor beta (ERβ) also known as NR3A2 is one of two main types of estrogen receptor—a nuclear receptor which is activated by the sex hormone estrogen. In humans ERβ is encoded by the ESR2 gene.

S100 calcium-binding protein A2 (S100A2) is a protein that in humans is encoded by the S100A2 gene and it is located on chromosome 1q21 with other S100 proteins.

Homeobox protein Hox-A3 is a protein that in humans is encoded by the HOXA3 gene.
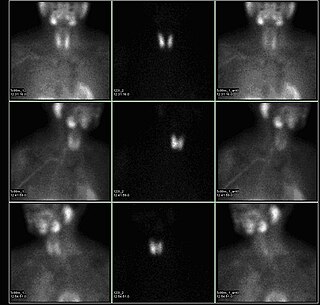
A sestamibi parathyroid scan is a procedure in nuclear medicine which is performed to localize parathyroid adenoma, which causes Hyperparathyroidism. Adequate localization of parathyroid adenoma allows the surgeon to use a minimally invasive surgical approach.
The fetal endocrine system is one of the first systems to develop during prenatal development of a human individual. The endocrine system arises from all three embryonic germ layers. The endocrine glands that produce the steroid hormones, such as the gonads and adrenal cortex, arise from the mesoderm. In contrast, endocrine glands that arise from the endoderm and ectoderm produce the amine, peptide, and protein hormones.
















