
A head injury is any injury that results in trauma to the skull or brain. The terms traumatic brain injury and head injury are often used interchangeably in the medical literature. Because head injuries cover such a broad scope of injuries, there are many causes—including accidents, falls, physical assault, or traffic accidents—that can cause head injuries.

Cerebral palsy (CP) is a group of movement disorders that appear in early childhood. Signs and symptoms vary among people and over time, but include poor coordination, stiff muscles, weak muscles, and tremors. There may be problems with sensation, vision, hearing, and speaking.

Colpocephaly is a cephalic disorder involving the disproportionate enlargement of the occipital horns of the lateral ventricles and is usually diagnosed early after birth due to seizures. It is a nonspecific finding and is associated with multiple neurological syndromes, including agenesis of the corpus callosum, Chiari malformation, lissencephaly, and microcephaly. Although the exact cause of colpocephaly is not known yet, it is commonly believed to occur as a result of neuronal migration disorders during early brain development, intrauterine disturbances, perinatal injuries, and other central nervous system disorders. Individuals with colpocephaly have various degrees of motor disabilities, visual defects, spasticity, and moderate to severe intellectual disability. No specific treatment for colpocephaly exists, but patients may undergo certain treatments to improve their motor function or intellectual disability.
Porencephaly is an extremely rare cephalic disorder involving encephalomalacia. It is a neurological disorder of the central nervous system characterized by cysts or cavities within the cerebral hemisphere. Porencephaly was termed by Heschl in 1859 to describe a cavity in the human brain. Derived from Greek roots, the word porencephaly means 'holes in the brain'. The cysts and cavities are more likely to be the result of destructive (encephaloclastic) cause, but can also be from abnormal development (malformative), direct damage, inflammation, or hemorrhage. The cysts and cavities cause a wide range of physiological, physical, and neurological symptoms. Depending on the patient, this disorder may cause only minor neurological problems, without any disruption of intelligence, while others may be severely disabled or die before the second decade of their lives. However, this disorder is far more common within infants, and porencephaly can occur both before or after birth.

Kernicterus is a bilirubin-induced brain dysfunction. The term was coined in 1904 by Christian Georg Schmorl. Bilirubin is a naturally occurring substance in the body of humans and many other animals, but it is neurotoxic when its concentration in the blood is too high, a condition known as hyperbilirubinemia. Hyperbilirubinemia may cause bilirubin to accumulate in the grey matter of the central nervous system, potentially causing irreversible neurological damage. Depending on the level of exposure, the effects range from clinically unnoticeable to severe brain damage and even death.

Neurotrauma, brain damage or brain injury (BI) is the destruction or degeneration of brain cells. Brain injuries occur due to a wide range of internal and external factors. In general, brain damage refers to significant, undiscriminating trauma-induced damage.
Hypotonia is a state of low muscle tone, often involving reduced muscle strength. Hypotonia is not a specific medical disorder, but a potential manifestation of many different diseases and disorders that affect motor nerve control by the brain or muscle strength. Hypotonia is a lack of resistance to passive movement, whereas muscle weakness results in impaired active movement. Central hypotonia originates from the central nervous system, while peripheral hypotonia is related to problems within the spinal cord, peripheral nerves and/or skeletal muscles. Severe hypotonia in infancy is commonly known as floppy baby syndrome. Recognizing hypotonia, even in early infancy, is usually relatively straightforward, but diagnosing the underlying cause can be difficult and often unsuccessful. The long-term effects of hypotonia on a child's development and later life depend primarily on the severity of the muscle weakness and the nature of the cause. Some disorders have a specific treatment but the principal treatment for most hypotonia of idiopathic or neurologic cause is physical therapy and/or occupational therapy for remediation.
Monoplegia is paralysis of a single limb, usually an arm. Common symptoms associated with monoplegic patients are weakness, numbness, and pain in the affected limb. Monoplegia is a type of paralysis that falls under hemiplegia. While hemiplegia is paralysis of half of the body, monoplegia is localized to a single limb or to a specific region of the body. Monoplegia of the upper limb is sometimes referred to as brachial monoplegia, and that of the lower limb is called crural monoplegia. Monoplegia in the lower extremities is not as common of an occurrence as in the upper extremities. Monoparesis is a similar, but less severe, condition because one limb is very weak, not paralyzed. For more information, see paresis.

Gray matter heterotopia is a neurological disorder caused by gray matter being located in an atypical location in the brain.

Intraventricular hemorrhage (IVH), also known as intraventricular bleeding, is a bleeding into the brain's ventricular system, where the cerebrospinal fluid is produced and circulates through towards the subarachnoid space. It can result from physical trauma or from hemorrhagic stroke.
Spastic quadriplegia, also known as spastic tetraplegia, is a subset of spastic cerebral palsy that affects all four limbs.
Neonatal encephalopathy (NE), previously known as neonatal hypoxic-ischemic encephalopathy, is defined as a encephalopathy syndrome with signs and symptoms of abnormal neurological function, in the first few days of life in an infant born after 35 weeks of gestation. In this condition there is difficulty initiating and maintaining respirations, a subnormal level of consciousness, and associated depression of tone, reflexes, and possibly seizures.Hypoxia refers to deficiency of oxygen, Ischemia refers to restriction in blood flow to the brain. The result is “encephalopathy” which refers to damaged brain cells. Encephalopathy is a nonspecific response of the brain to injury which may occur via multiple methods, but is commonly caused by birth asphyxia, leading to cerebral hypoxia.
Dyskinetic cerebral palsy (DCP) is a subtype of cerebral palsy (CP) and is characterized by impaired muscle tone regulation, coordination and movement control. Dystonia and choreoathetosis are the two most dominant movement disorders in patients with DCP.

Athetoid cerebral palsy, or dyskinetic cerebral palsy, is a type of cerebral palsy primarily associated with damage, like other forms of CP, to the basal ganglia in the form of lesions that occur during brain development due to bilirubin encephalopathy and hypoxic–ischemic brain injury. Unlike spastic or ataxic cerebral palsies, ADCP is characterized by both hypertonia and hypotonia, due to the affected individual's inability to control muscle tone. Clinical diagnosis of ADCP typically occurs within 18 months of birth and is primarily based upon motor function and neuroimaging techniques. While there are no cures for ADCP, some drug therapies as well as speech, occupational therapy, and physical therapy have shown capacity for treating the symptoms.
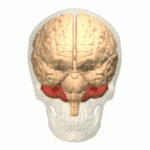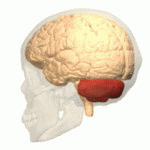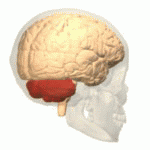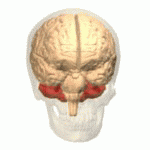
Ataxic cerebral palsy is clinically in approximately 5–10% of all cases of cerebral palsy, making it the least frequent form of cerebral palsy diagnosed. Ataxic cerebral palsy is caused by damage to cerebellar structures, differentiating it from the other two forms of cerebral palsy, which are spastic cerebral palsy and dyskinetic cerebral palsy.

Spastic cerebral palsy is the type of cerebral palsy characterized by spasticity or high muscle tone often resulting in stiff, jerky movements. Cases of spastic CP are further classified according to the part or parts of the body that are most affected. Such classifications include spastic diplegia, spastic hemiplegia, spastic quadriplegia, and in cases of single limb involvement, spastic monoplegia.

Ulegyria is a diagnosis used to describe a specific type of cortical scarring in the deep regions of the sulcus that leads to distortion of the gyri. Ulegyria is identified by its characteristic "mushroom-shaped" gyri, in which scarring causes shrinkage and atrophy in the deep sulcal regions while the surface gyri are spared. This condition is most often caused by hypoxic-ischemic brain injury in the perinatal period. The effects of ulegyria can range in severity, although it is most commonly associated with cerebral palsy, mental retardation and epilepsy. N.C. Bresler was the first to view ulegyria in 1899 and described this abnormal morphology in the brain as “mushroom-gyri." Although ulegyria was first identified in 1899, there is still limited information known or reported about the condition.

Yoon Bo-hyun (Korean: 윤보현) is a South Korean physician and scientist in the medical area of obstetrics and gynecology. He researches in the area of preterm births, intra-amniotic infection or inflammation and fetal damage. For his theoretical and clinical academic achievements he received the Top Scientist and Technologist Award of Korea in 2012.

A general movements assessment is a type of medical assessment used in the diagnosis of cerebral palsy, and is particularly used to follow up high-risk neonatal cases. The general movements assessment involves measuring movements that occur spontaneously among those less than four months of age and appears to be most accurate test for the condition.
Cranial ultrasound is a technique for scanning the brain using high-frequency sound waves. It is used almost exclusively in babies because their fontanelle provides an "acoustic window". A different form of ultrasound-based brain scanning, transcranial Doppler, can be used in any age group. This uses Doppler ultrasound to assess blood flow through the major arteries in the brain, and can scan through bone. It is not usual for this technique to be referred to simply as "cranial ultrasound". Additionally, cranial ultrasound can be used for intra-operative imaging in adults undergoing neurosurgery once the skull has been opened, for example to help identify the margins of a tumour.













