Related Research Articles
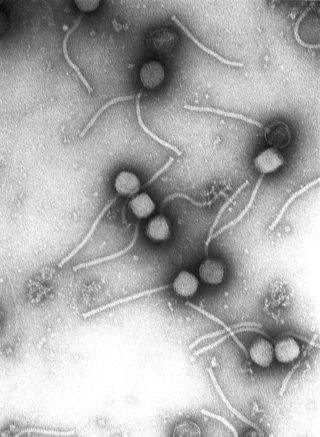
Virology is the scientific study of biological viruses. It is a subfield of microbiology that focuses on their detection, structure, classification and evolution, their methods of infection and exploitation of host cells for reproduction, their interaction with host organism physiology and immunity, the diseases they cause, the techniques to isolate and culture them, and their use in research and therapy.

The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the ligand to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.

HIV tests are used to detect the presence of the human immunodeficiency virus (HIV), the virus that causes HIV/AIDS, in serum, saliva, or urine. Such tests may detect antibodies, antigens, or RNA.
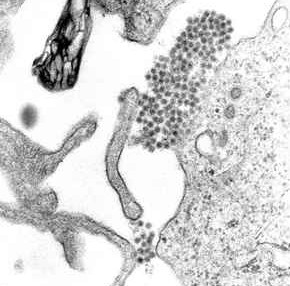
Dengue virus (DENV) is the cause of dengue fever. It is a mosquito-borne, single positive-stranded RNA virus of the family Flaviviridae; genus Flavivirus. Four serotypes of the virus have been found, and a reported fifth has yet to be confirmed, all of which can cause the full spectrum of disease. Nevertheless, the mainstream scientific community's understanding of dengue virus may be simplistic as, rather than distinct antigenic groups, a continuum appears to exist. This same study identified 47 strains of dengue virus. Additionally, coinfection with and lack of rapid tests for Zika virus and chikungunya complicate matters in real-world infections.

Thymidine kinase is an enzyme, a phosphotransferase : 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, EC 2.7.1.21. It can be found in most living cells. It is present in two forms in mammalian cells, TK1 and TK2. Certain viruses also have genetic information for expression of viral thymidine kinases. Thymidine kinase catalyzes the reaction:
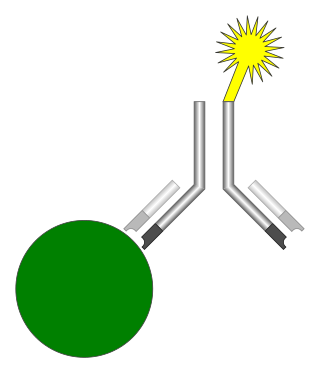
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.
Hemagglutination, or haemagglutination, is a specific form of agglutination that involves red blood cells (RBCs). It has two common uses in the laboratory: blood typing and the quantification of virus dilutions in a haemagglutination assay.

Influenza C virus is the only species in the genus Gammainfluenzavirus, in the virus family Orthomyxoviridae, which like other influenza viruses, causes influenza.
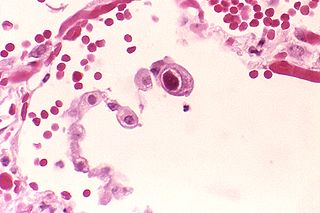
Human betaherpesvirus 5, also called human cytomegalovirus (HCMV,HHV-5), is a species of virus in the genus Cytomegalovirus, which in turn is a member of the viral family known as Herpesviridae or herpesviruses. It is also commonly called CMV. Within Herpesviridae, HCMV belongs to the Betaherpesvirinae subfamily, which also includes cytomegaloviruses from other mammals. CMV is a double-stranded DNA virus.

Antibody-dependent enhancement (ADE), sometimes less precisely called immune enhancement or disease enhancement, is a phenomenon in which binding of a virus to suboptimal antibodies enhances its entry into host cells, followed by its replication. The suboptimal antibodies can result from natural infection or from vaccination. ADE may cause enhanced respiratory disease, but is not limited to respiratory disease. It has been observed in HIV, RSV, and Dengue virus and is monitored for in vaccine development.

Anti-double stranded DNA (Anti-dsDNA) antibodies are a group of anti-nuclear antibodies (ANA) the target antigen of which is double stranded DNA. Blood tests such as enzyme-linked immunosorbent assay (ELISA) and immunofluorescence are routinely performed to detect anti-dsDNA antibodies in diagnostic laboratories. They are highly diagnostic of systemic lupus erythematosus (SLE) and are implicated in the pathogenesis of lupus nephritis.
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy.
Virus quantification is counting or calculating the number of virus particles (virions) in a sample to determine the virus concentration. It is used in both research and development (R&D) in academic and commercial laboratories as well as in production situations where the quantity of virus at various steps is an important variable that must be monitored. For example, the production of virus-based vaccines, recombinant proteins using viral vectors, and viral antigens all require virus quantification to continually monitor and/or modify the process in order to optimize product quality and production yields and to respond to ever changing demands and applications. Other examples of specific instances where viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization, and adaptation of methods to cell culture.
NS1 antigen test is a test for dengue, introduced in 2006. It allows rapid detection on the first day of fever, before antibodies appear some 5 or more days later. It has been adopted for use in some 40 nations. The method of detection is through enzyme-linked immunosorbent assay. India has introduced in 2010 the NS1 test costing 1,600 rupees at a private hospital in Mumbai.

Indirect immunoperoxidase assay (IPA) is a laboratory technique used to detect and titrate viruses that do not cause measurable cytopathic effects and cannot be measured by classical plaque assays. These viruses include human coronavirus 229E and OC43.
Falcon aviadenovirus A is an avian adenovirus that infects birds of the genus Falco, commonly called falcons. The virus was first discovered in 1996 in an epizootic of inclusion body hepatitis and enteritis in aplomado and peregrine falcons. It can also infect orange-breasted falcons, taitas, merlins, and American kestrels.
Jamestown Canyon encephalitis is an infectious disease caused by the Jamestown Canyon virus, an orthobunyavirus of the California serogroup. It is mainly spread during the summer by different mosquito species in the United States and Canada.
Thymidine kinase is an enzyme, a phosphotransferase : 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, EC 2.7.1.21 that catalyzes the reaction:
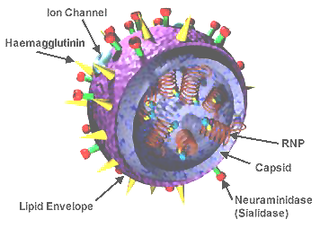
Influenza D virus is a species in the virus genus Deltainfluenzavirus, in the family Orthomyxoviridae, that causes influenza.

Reporter virus particles (RVPs) are replication-incompetent virus particles engineered to express one or more reporter genes upon infecting susceptible cells. Since the RVP genome lacks genes essential for viral replication, RVPs are capable of only a single round of infection. Thus they are safe to work with under BSL-2 conditions, enabling the study of highly pathogenic viruses using standard laboratory facilities. Expression of a reporter such as luciferase can provide a quantitative readout of infection. With proper design and quality control, RVPs remain stable under common assay conditions and yield reproducible results that correlate with those obtained from live virus. These qualities make RVPs a safer and faster alternative to plaque assays, and especially well-suited for high-throughput applications. RVPs offer flexibility for different uses, as they are antigenically identical to wild-type virus, and can be engineered with various proteins or express mutant envelopes to study infectivity or antigenicity.
References
- ↑ Schmidt, N J; Dennis, J; Lennette, E H (July 1976). "Plaque reduction neutralization test for human cytomegalovirus based upon enhanced uptake of neutral red by virus-infected cells". Journal of Clinical Microbiology. 4 (1): 61–66. doi:10.1128/jcm.4.1.61-66.1976. ISSN 0095-1137. PMC 274391 . PMID 182716.
- ↑ Klasse, P. J. (2014-09-09). "Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives". Advances in Biology. 2014: 1–24. doi: 10.1155/2014/157895 . PMC 4835181 . PMID 27099867.
- ↑ Schmidt, N J; J Dennis; E H Lennette (July 1976). "Plaque reduction neutralization test for human cytomegalovirus based upon enhanced uptake of neutral red by virus-infected cells" (PDF). Journal of Clinical Microbiology. 4 (1): 61–66. doi:10.1128/jcm.4.1.61-66.1976. ISSN 0095-1137. PMC 274391 . PMID 182716.
- ↑ Thomas, Stephen J.; Ananda Nisalak; Kathryn B. Anderson; Daniel H. Libraty; Siripen Kalayanarooj; David W. Vaughn; Robert Putnak; Robert V. Gibbons; Richard Jarman; Timothy P. Endy (November 2009). "Dengue Plaque Reduction Neutralization Test (PRNT) in Primary and Secondary Dengue Virus Infections: How Alterations in Assay Conditions Impact Performance". The American Journal of Tropical Medicine and Hygiene. 81 (5): 825–833. doi:10.4269/ajtmh.2009.08-0625. ISSN 0002-9637. PMC 2835862 . PMID 19861618.
- ↑ Ratnam, S; V Gadag; R West; J Burris; E Oates; F Stead; N Bouilianne (1995-04-01). "Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody". J. Clin. Microbiol. 33 (4): 811–815. doi: 10.1128/JCM.33.4.811-815.1995 . PMC 228046 . PMID 7790442.
- ↑ Mukherjee, S.; Dowd, K. A.; Manhart, C. J.; Ledgerwood, J. E.; Durbin, A. P.; Whitehead, S. S.; Pierson, T. C. (2014). "Mechanism and Significance of Cell Type-Dependent Neutralization of Flaviviruses". Journal of Virology. 88 (13): 7210–7220. doi:10.1128/JVI.03690-13. ISSN 0022-538X. PMC 4054442 . PMID 24741083.