
Poison dart frog is the common name of a group of frogs in the family Dendrobatidae which are native to tropical Central and South America. These species are diurnal and often have brightly colored bodies. This bright coloration is correlated with the toxicity of the species, making them aposematic. Some species of the family Dendrobatidae exhibit extremely bright coloration along with high toxicity, while others have cryptic coloration with minimal to no amount of observed toxicity. The species that have great toxicity derive this feature from their diet of ants, mites and termites. However, other species that exhibit cryptic coloration, and low to no amounts of toxicity, eat a much larger variety of prey. Many species of this family are threatened due to human infrastructure encroaching on their habitats.

Batrachotoxin (BTX) is an extremely potent cardio- and neurotoxic steroidal alkaloid found in certain species of beetles, birds, and frogs. The name is from the Greek word βάτραχος, bátrachos, 'frog'. Structurally-related chemical compounds are often referred to collectively as batrachotoxins. In certain frogs, this alkaloid is present mostly on the skin. Such frogs are among those used for poisoning darts. Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and prevents them from closing, resulting in paralysis and death. No antidote is known.

Cerberin is a type of cardiac glycoside, a steroidal class found in the seeds of the dicotyledonous angiosperm genus Cerbera; including the suicide tree and the sea mango. This class includes digitalis-like agents, channel-blockers that as a group have found historic uses as cardiac treatments, but which at higher doses are extremely toxic; in the case of cerberin, consumption of the C. odollam results in poisoning with presenting nausea, vomiting, and abdominal pain, often leading to death. The natural product has been structurally characterized, its toxicity is clear—it is often used as an intentional human poison in third-world countries, and accidental poisonings with fatalities have resulted from individuals even indirectly consuming the agent—but its potentially therapeutic pharmacologic properties are very poorly described.

The strawberry poison frog, strawberry poison-dart frog or blue jeans poison frog is a species of small poison dart frog found in Central America. It is common throughout its range, which extends from eastern central Nicaragua through Costa Rica and northwestern Panamá. The species is often found in humid lowlands and premontane forest, but large populations are also found in disturbed areas such as plantations. The strawberry poison frog is perhaps most famous for its widespread variation in coloration, comprising approximately 15–30 color morphs, most of which are presumed to be true-breeding. O. pumilio, while not the most poisonous of the dendrobatids, is the most toxic member of its genus.

The dyeing dart frog, cobalt poison frog, dyeing poison dart frog, tinc, or dyeing poison frog is a species of poison dart frog. It is among the largest species, reaching lengths of 50 mm (2.0 in). This species is distributed throughout the eastern portion of the Guiana Shield and Venezuela, including parts of Guyana, Suriname, Brazil, and nearly all of French Guiana.

The golden poison frog, also known as the golden dart frog or golden poison arrow frog, is a poison dart frog endemic to the rainforests of Colombia. The golden poison frog has become endangered due to habitat destruction within its naturally limited range. Despite its small size, this frog is among the most poisonous animals on the planet.

Phyllobates is a genus of poison dart frogs native to Central and South America, from Nicaragua to Colombia. There are 3 different Colombian species of Phyllobates, considered highly toxic species due to the poison they contain in the wild.

Phyllobates aurotaenia is a member of the frog family Dendrobatidae, which are found in the tropical environments of Central and South America. First described by zoologist George Albert Boulenger in 1913, P. aurotaenia is known for being the third most poisonous frog in the world. It is the smallest of the poison dart frogs in the Phyllobates genus and is endemic to the Pacific coast of Colombia.

Calciseptine (CaS) is a natural neurotoxin isolated from the black mamba Dendroaspis p. polylepis venom. This toxin consists of 60 amino acids with four disulfide bonds. Calciseptine specifically blocks L-type calcium channels, but not other voltage-dependent Ca2+ channels such as N-type and T-type channels.

Oophaga sylvatica, sometimes known as its Spanish name diablito, is a species of frog in the family Dendrobatidae found in Southwestern Colombia and Northwestern Ecuador. Its natural habitat is lowland and submontane rainforest; it can, however, survive in moderately degraded areas, at least in the more humid parts of its range. It is a very common frog in Colombia, but has disappeared from much of its Ecuadorian range. It is threatened by habitat loss (deforestation) and agricultural pollution and sometimes seen in the international pet trade.

The Golfodulcean poison frog or Golfodulcean poison-arrow frog is a species of frog in the family Dendrobatidae endemic to Costa Rica.

Pumiliotoxins (PTXs), are one of several toxins found in the skin of poison dart frogs. The frog species, P. bibronii also produces PTXs to deter predators. Closely related, though more toxic, are allopumiliotoxins, (aPTXs). Other toxins found in the skin of poison frogs include decahydroquinolines (DHQs), izidines, coccinellines, and spiropyrrolizidine alkaloids. Pumiliotoxins are very poisonous in high concentrations. Pumiliotoxins are much weaker than batrachotoxins, ranging between 100 and 1000 times less poisonous. There are three different types of this toxin: A, B and C, of which toxins A and B are more toxic than C. Pumiliotoxins interfere with muscle contraction by affecting calcium channels, causing partial paralysis, difficulty moving, hyperactivity, or death. The median lethal dose of pumiliotoxins A and B is 50 µg / mouse, 20 µg / mouse respectively, while the amount of pumiliotoxin is 200 µg / frog.
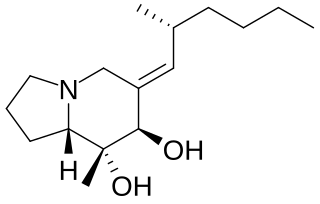
Allopumiliotoxin 267A is a toxin found in the skin of several poison frogs of the family Dendrobates. It is a member of the class of compounds known as allopumiliotoxins. The frogs produce the toxin by modifying the original version, pumiliotoxin 251D. It has been tested on mice and found to be five times more potent than the former version. It has been produced synthetically through a variety of different routes.

Allopumiliotoxins are a structural division in the pumiliotoxin-A class of alkaloids. The compounds of the pumiliotoxin-A class are primarily found in the skins of frogs, toads, and other amphibians and are used as a chemical defense mechanism to ward off predators, microorganisms, and ectoparasites. The compounds were originally discovered in neotropical dendrobatid frogs, but are also found in the mantellid frogs of Madagascar, myobatrachid frogs of Australia, and bufonid toad of South America. Frogs possessing this defense mechanism have aposematic coloring.

Histrionicotoxins are a group of related toxins found in the skin of poison frogs from the family Dendrobatidae, notably Oophaga histrionica, which are native to Colombia. It is likely that, as with other poison frog alkaloids, histrionicotoxins are not manufactured by the amphibians, but absorbed from insects in their diet and stored in glands in their skin. They are notably less toxic than other alkaloids found in poison frogs, yet their distinct structure acts as a neurotoxin by non-competitive inhibition of nicotinic acetylcholine receptors.

Adelphobates is a small genus of poison dart frogs. They are found in the central and lower Amazon basin of Peru and Brazil, possibly Bolivia. It was originally erected as a sister group to the Dendrobates and Oophaga genera. The validity of the genus is still being discussed, with the alternative being "Dendrobates galactonotus group" within Dendrobates. One species originally placed in this genus as Adelphobates captivus has since been moved to the genus Excidobates erected in 2008.
Jingzhaotoxin proteins are part of a venom secreted by Chilobrachys jingzhao, the Chinese tarantula. and act as neurotoxins. There are several subtypes of jingzhaotoxin, which differ in terms of channel selectivity and modification characteristics. All subspecies act as gating modifiers of sodium channels and/or, to a lesser extent, potassium channels.

Neosaxitoxin (NSTX) is included, as other saxitoxin-analogs, in a broad group of natural neurotoxic alkaloids, commonly known as the paralytic shellfish toxins (PSTs). The parent compound of PSTs, saxitoxin (STX), is a tricyclic perhydropurine alkaloid, which can be substituted at various positions, leading to more than 30 naturally occurring STX analogues. All of them are related imidazoline guanidinium derivatives.

Dioscorine is an alkaloid toxin isolated from the tubers of tropical yam on several continents. It has been used as a monkey poison in some African countries, and as an arrow poison to aid in hunting in several parts of Asia. It was first isolated from Dioscorea hirsute by Boorsma in 1894 and obtained in a crystalline form by Schutte in 1897, and has since been found in other Dioscorea species. Dioscorine is a neurotoxin that acts by blocking the nicotinic acetylcholine receptor. Dioscorine is generally isolated in tandem with other alkaloids such as dioscin but is usually the most potent toxin in the mixture. It is a convulsant, producing symptoms similar to picrotoxin, with which it shares a similar mechanism of action.
Gambierol is a marine polycyclic ether toxin which is produced by the dinoflagellate Gambierdiscus toxicus. Gambierol is collected from the sea at the Rangiroa Peninsula in French Polynesia. The toxins are accumulated in fish through the food chain and can therefore cause human intoxication. The symptoms of the toxicity resemble those of ciguatoxins, which are extremely potent neurotoxins that bind to voltage-sensitive sodium channels and alter their function. These ciguatoxins cause ciguatera fish poisoning. Because of the resemblance, there is a possibility that gambierol is also responsible for ciguatera fish poisoning. Because the natural source of gambierol is limited, biological studies are hampered. Therefore, chemical synthesis is required.

















