
Buckwheat or common buckwheat is a flowering plant in the knotweed family Polygonaceae cultivated for its grain-like seeds and as a cover crop. Buckwheat originated around the 6th millennium BCE in the region of what is now Yunnan Province in southwestern China. The name "buckwheat" is used for several other species, such as Fagopyrum tataricum, a domesticated food plant raised in Asia.
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

Quercitron is a yellow natural dye obtained from the bark of the Eastern Black Oak, a forest tree indigenous in North America. It was formerly called Dutch pink, English pink, or Italian pink.

The genus Fagopyrum is in the flowering plant family Polygonaceae. It includes some important food plants, such as F. esculentum (buckwheat) and F. tataricum. The genus is native to the Indian subcontinent, much of Indochina, and central and southeastern China. Species have been widely introduced elsewhere, throughout the Holarctic and parts of Africa and South America.
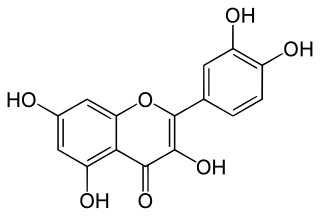
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.

Fagopyrum tataricum, also known as Tartary buckwheat, green buckwheat, ku qiao, Tatar buckwheat, or bitter buckwheat, is a domesticated food plant in the genus Fagopyrum in the family Polygonaceae. With another species in the same genus, common buckwheat, it is often counted as a cereal, but the buckwheats are not closely related to true cereals.

Rutin is the glycoside combining the flavonol quercetin and the disaccharide rutinose. It is a flavonoid glycoside found in a wide variety of plants, including citrus.

2-Nonenal is an unsaturated aldehyde. The colorless liquid is an important aroma component of aged beer and buckwheat and insoluble in water.
The enzyme quercitrinase (EC 3.2.1.66) catalyzes the following chemical reaction:

Orientin is a flavone, a chemical flavonoid-like compound. It is the 8-C glucoside of luteolin.

Hyperoside is a chemical compound. It is the 3-O-galactoside of quercetin.
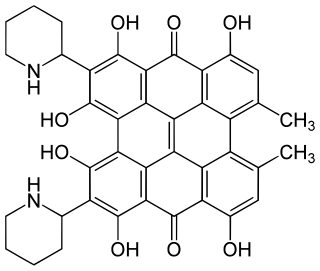
Fagopyrin is a term used for several closely related naturally occurring substances in the buckwheat plant. Their chemical structure contains a naphthodianthrone skeleton similar to that of hypericin.

Grandinin is an ellagitannin. It can be found in Melaleuca quinquenervia leaves and in oaks species like the North American white oak and European red oak. It shows antioxydant activity. It is an astringent compound. It is also found in wine, red or white, aged in oak barrels.
Tergallic acids are trimers of gallic acid, often found naturally in the form of glycosides. Tergallic acid O- or C-glucosides that can be found in acorns of several Quercus (oak) species. The dehydrated tergallic acid C-glucoside and tergallic acid O-glucoside can be characterised in the acorns of Quercus macrocarpa. Dehydrated tergallic-C-glucoside can be found in the cork from Quercus suber.
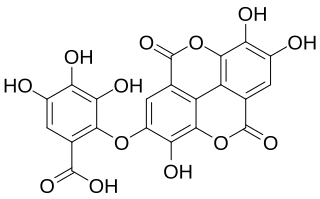
Valoneic acid dilactone is a hydrolysable tannin that can be isolated from the heartwood of Shorea laevifolia and in oaks species like the North American white oak and European red oak.

Avicularin is a bio-active flavonol isolated from a number of plants including Polygonum aviculare, Rhododendron aureum and Taxillus kaempferi.

Taxillus kaempferi is a parasitic plant species in the genus Taxillus found in China, Bhutan and Japan. Its host is Pinus thunbergii.

Taxillusin is a flavonol found in the parasitic plant Taxillus kaempferi. It is a galloylated 3-O-glucoside of quercetin.

Fagopyrum cymosum, also known as tall buckwheat, is a domesticated plant used in traditional Chinese medicine, for animal feed, and as an ornamental plant. It is native to much of China, and to Bhutan, Nepal, India, Burma, and Vietnam.
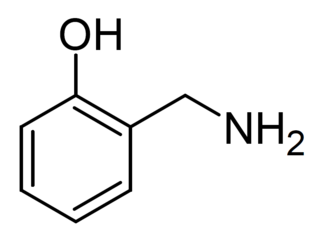
2-Hydroxybenzylamine is a natural product found in Himalayan tartary buckwheat. It acts as an antioxidant and scavanger of free radicals and isolevuglandins and is sold as a dietary supplement.


















