
A myelodysplastic syndrome (MDS) is one of a group of cancers in which immature blood cells in the bone marrow do not mature, and as a result, do not develop into healthy blood cells. Early on, no symptoms typically are seen. Later, symptoms may include fatigue, shortness of breath, bleeding disorders, anemia, or frequent infections. Some types may develop into acute myeloid leukemia.
Aplastic anemia (AA) is a severe hematologic condition in which the body fails to make blood cells in sufficient numbers. Aplastic anemia is associated with cancer and various cancer syndromes. Blood cells are produced in the bone marrow by stem cells that reside there. Aplastic anemia causes a deficiency of all blood cell types: red blood cells, white blood cells, and platelets.

Immune thrombocytopenic purpura (ITP), also known as idiopathic thrombocytopenic purpura or immune thrombocytopenia, is a type of thrombocytopenic purpura characterized by a low platelet count in the absence of other causes, and accompanied by a red-purple rash called purpura. It leads to an increased risk of bleeding. ITP manifests in two distinct clinical syndromes: an acute form observed in children, and chronic conditions observed in adults. The acute form often follows an infection and typically resolves within two months, while chronic immune thrombocytopenia persists for longer than six months and its specific cause is unknown.

Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, life-threatening disease of the blood characterized by destruction of red blood cells by the complement system, a part of the body's innate immune system. This destructive process occurs due to deficiency of the red blood cell surface protein DAF, which normally inhibits such immune reactions. Since the complement cascade attacks the red blood cells within the blood vessels of the circulatory system, the red blood cell destruction (hemolysis) is considered an intravascular hemolytic anemia. There is ongoing research into other key features of the disease, such as the high incidence of venous blood clot formation. Research suggests that PNH thrombosis is caused by both the absence of GPI-anchored complement regulatory proteins on PNH platelets and the excessive consumption of nitric oxide (NO).
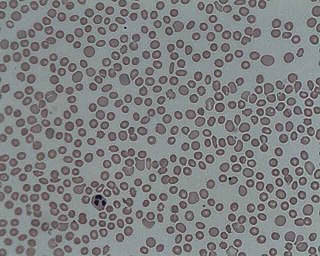
In hematology, thrombocytopenia is a condition characterized by abnormally low levels of platelets in the blood. Low levels of platelets in turn may lead to prolonged or excessive bleeding. It is the most common coagulation disorder among intensive care patients and is seen in a fifth of medical patients and a third of surgical patients.
Pancytopenia is a medical condition in which there is significant reduction in the number of almost all blood cells.
Mean platelet volume (MPV) is a machine-calculated measurement of the average size of platelets found in blood and is typically included in blood tests as part of the CBC. Since the average platelet size is larger when the body is producing increased numbers of platelets, the MPV test results can be used to make inferences about platelet production in bone marrow or platelet destruction problems.
Aplasia is a birth defect where an organ or tissue is wholly or largely absent. It is caused by a defect in a developmental process.
Reticulocytopenia is the medical term for an abnormal decrease in circulating red blood cell precursors (reticulocytes) that can lead to anemia due to resulting low red blood cell (erythrocyte) production. Reticulocytopenia may be an isolated finding or it may not be associated with abnormalities in other hematopoietic cell lineages such as those that produce white blood cells (leukocytes) or platelets (thrombocytes), a decrease in all three of these lineages is referred to as pancytopenia.

Eltrombopag, sold under the brand name Promacta among others, is a medication used to treat thrombocytopenia and severe aplastic anemia. Eltrombopag is sold under the brand name Revolade outside the US and is marketed by Novartis. It is a thrombopoietin receptor agonist. It is taken by mouth.

Congenital amegakaryocytic thrombocytopenia (CAMT) is a rare autosomal recessive bone marrow failure syndrome characterized by severe thrombocytopenia, which can progress to aplastic anemia and leukemia. CAMT usually manifests as thrombocytopenia in the initial month of life or in the fetal phase. Typically CAMPT presents with petechiae, cerebral bleeds, recurrent rectal bleeding, or pulmonary hemorrhage.

Drug-induced autoimmune hemolytic anemia also known as Drug-induced immune hemolytic anemia (DIIHA) is a rare cause of hemolytic anemia. It is difficult to differentiate from other forms of anemia which can lead to delays in diagnosis and treatment. Many different types of antibiotics can cause DIIHA and discontinuing the offending medication is the first line of treatment. DIIHA has is estimated to affect one to two people per million worldwide.
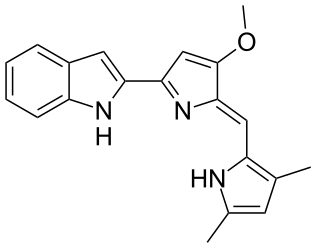
Obatoclax mesylate, also known as GX15-070, is an experimental drug for the treatment of various types of cancer. It was discovered by Gemin X, which was acquired by Cephalon, which has since been acquired by Teva Pharmaceuticals. Several Phase II clinical trials were completed that investigated use of obatoclax in the treatment of leukemia, lymphoma, myelofibrosis, and mastocytosis.

Fostamatinib, sold under the brand names Tavalisse and Tavlesse, is a tyrosine kinase inhibitor medication for the treatment of chronic immune thrombocytopenia (ITP). The drug is administered by mouth.
Olokizumab (OKZ) sold under the name Artlegia, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis and COVID-19. It is a humanized monoclonal antibody against the interleukin-6 (IL-6). IL-6 is a cytokine that plays an important role in immune response and is implicated in the pathogenesis of many diseases. Olokizumab is the first interleukin-6 (IL-6) inhibitor approved for treatment of rheumatoid arthritis which blocks directly cytokine instead of its receptor. Olokizumab specifically binds to IL-6 at Site 3, blocking IL-6 ability to form hexameric complex. Olokizumab was developed by R-Pharm group, and was launched in 2020.
Bone marrow failure occurs in individuals who produce an insufficient amount of red blood cells, white blood cells or platelets. Red blood cells transport oxygen to be distributed throughout the body's tissue. White blood cells fight off infections that enter the body. Bone marrow also contains platelets, which trigger clotting, and thus help stop the blood flow when a wound occurs.
Neal Stuart Young is an American physician and researcher, chief of the Hematology Branch of the National Institutes of Health (NIH), and Director of the Center for Human Immunology at the NIH in Bethesda, Maryland. He is primarily known for his work in the pathophysiology and treatment of aplastic anemia, and is also known for his contributions to the pathophysiology of parvovirus B19 infection.

Tislelizumab, sold under the brand name Tevimbra among others, is a humanized monoclonal antibody directed against PD-1. It prevents PD-1 from binding to the ligands PD-L1 and PD-L2. It is designed to bind less to Fc gamma receptors. It is being developed by BeiGene.

Selinexor sold under the brand name Xpovio among others, is a selective inhibitor of nuclear export used as an anti-cancer medication. It works by blocking the action of exportin 1 and thus blocking the transport of several proteins involved in cancer-cell growth from the cell nucleus to the cytoplasm, which ultimately arrests the cell cycle and leads to apoptosis. It is the first drug with this mechanism of action.

Abrocitinib, sold under the brand name Cibinqo, is a medication used for the treatment of atopic dermatitis (eczema). It is a Janus kinase inhibitor and it was developed by Pfizer. It is taken by mouth.












