
Caspofungin is a lipopeptide antifungal drug from Merck & Co., Inc.. It is a member of a class of antifungals termed the echinocandins. It works by inhibiting the enzyme (1→3)-β-D-glucan synthase and thereby disturbing the integrity of the fungal cell wall.

Anidulafungin (INN) is a semisynthetic echinocandin used as an antifungal drug. It was previously known as LY303366. It may also have application in treating invasive Aspergillus infection when used in combination with voriconazole. It is a member of the class of antifungal drugs known as the echinocandins; its mechanism of action is by inhibition of (1→3)-β-D-glucan synthase, an enzyme important to the synthesis of the fungal cell wall.
Ustekinumab, sold under the brand name Stelara among others, is a monoclonal antibody medication developed by Janssen Pharmaceuticals, for the treatment of Crohn's disease, ulcerative colitis, plaque psoriasis and psoriatic arthritis, targeting both IL-12 and IL-23.

Tralokinumab sold under the brand names Adtralza (EU/UK) and Adbry (US) among others, is a human monoclonal antibody used for the treatment of atopic dermatitis. Tralokinumab targets the cytokine interleukin 13.
Anifrolumab, sold under the brand name Saphnelo, is a monoclonal antibody used for the treatment of systemic lupus erythematosus. It binds to the type I interferon receptor, blocking the activity of type I interferons such as interferon-α and interferon-β.
Enfortumab vedotin, sold under the brand name Padcev, is an antibody-drug conjugate used for the treatment of urothelial cancer. It is a nectin-4-directed antibody and microtubule inhibitor conjugate. Enfortumab refers to the monoclonal antibody part, and vedotin refers to the payload drug (MMAE) and the linker.
Inebilizumab, sold under the brand name Uplizna, is a medication for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adults. Inebilizumab is a humanized mAb that binds to and depletes CD19+ B cells including plasmablasts and plasma cells.
Mirikizumab, sold under the brand name Omvoh, is a monoclonal antibody used for the treatment of ulcerative colitis. It is designed to attach to interleukin-23 (IL-23) and block its activity.
Bimekizumab, sold under the brand name Bimzelx, is a humanized anti-IL17A, anti-IL-17F, and anti-IL17AF monoclonal antibody that is used to treat plaque psoriasis, psoriatic arthritis, axial spondyloarthritis, and ankylosing spondylitis.
Sutimlimab, sold under the brand name Enjaymo, is a monoclonal antibody that is used to treat adults with cold agglutinin disease (CAD). It is given by intravenous infusion. Sutimlimab prevents complement-enhanced activation of autoimmune human B cells in vitro.
Tafasitamab, sold under the brand name Monjuvi, is a medication used in combination with lenalidomide for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL).
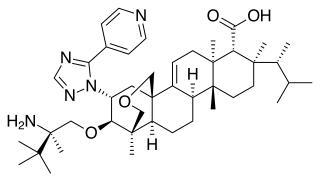
Ibrexafungerp, sold under the brand name Brexafemme, is an antifungal medication used to treat vulvovaginal candidiasis (VVC). It is taken orally. It is also currently undergoing clinical trials for other indications via an intravenous (IV) formulation. An estimated 75% of women will have at least one episode of VVC and 40 to 45% will have two or more episodes in their lifetime.

Zilucoplan, sold under the brand name Zilbrysq, is a medication used for the treatment of generalized myasthenia gravis. It is a complement inhibitor that is injected subcutaneously.
Avalglucosidase alfa, sold under the brand name Nexviazyme, is an enzyme replacement therapy medication used for the treatment of glycogen storage disease type II.
Mosunetuzumab, sold under the brand name Lunsumio, is a monoclonal antibody used for the treatment of follicular lymphoma. It bispecifically binds CD20 and CD3 to engage T-cells. It was developed by Genentech.
Teclistamab, sold under the brand name Tecvayli, is a human bispecific monoclonal antibody used for the treatment of relapsed and refractory multiple myeloma. It is a bispecific antibody that targets the CD3 receptor expressed on the surface of T-cells and B-cell maturation antigen (BCMA), which is expressed on the surface of malignant multiple myeloma B-lineage cells.
Spesolimab, sold under the brand name Spevigo, is a monoclonal antibody used for the treatment of generalized pustular psoriasis (GPP). It is an interleukin-36 receptor (IL-36R) antagonist. It is given via injection into a vein.

Futibatinib, sold under the brand name Lytgobi, is an anti-cancer medication used for the treatment of cholangiocarcinoma. It is a kinase inhibitor. It is taken by mouth.
Epcoritamab, sold under the brand name Epkinly, is a monoclonal antibody anticancer medication used for the treatment of diffuse large B-cell lymphoma. Epcoritamab is a bispecific CD20-directed CD3 T-cell engager. Epcoritamab was co-developed by AbbVie and Genmab.
Rozanolixizumab, sold under the brand name Rystiggo, is a monoclonal antibody used for the treatment of myasthenia gravis. Rozanolixizumab is a humanized and chimeric monoclonal antibody; and is a neonatal Fc receptor blocker.






