
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitute 1% of all described fungal species.

Saccharomyces cerevisiae is a species of yeast. The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been originally isolated from the skin of grapes. It is one of the most intensively studied eukaryotic model organisms in molecular and cell biology, much like Escherichia coli as the model bacterium. It is the microorganism behind the most common type of fermentation. S. cerevisiae cells are round to ovoid, 5–10 μm in diameter. It reproduces by budding.
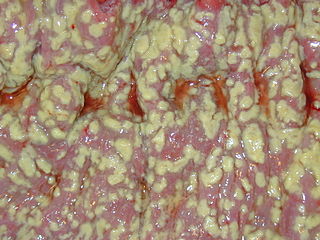
Clostridioides difficile infection , also known as Clostridium difficile infection, is a symptomatic infection due to the spore-forming bacterium Clostridioides difficile. Symptoms include watery diarrhea, fever, nausea, and abdominal pain. It makes up about 20% of cases of antibiotic-associated diarrhea. Antibiotics can contribute to detrimental changes in gut microbiota; specifically, they decrease short-chain fatty acid absorption which results in osmotic, or watery, diarrhea. Complications may include pseudomembranous colitis, toxic megacolon, perforation of the colon, and sepsis.

Probiotics are live microorganisms promoted with claims that they provide health benefits when consumed, generally by improving or restoring the gut microbiota. Probiotics are considered generally safe to consume, but may cause bacteria-host interactions and unwanted side effects in rare cases. There is some evidence that probiotics are beneficial for some conditions, but there is little evidence for many of the health benefits claimed for them.

Fungemia is the presence of fungi or yeasts in the blood. The most common type, also known as candidemia, candedemia, or systemic candidiasis, is caused by Candida species; candidemia is also among the most common bloodstream infections of any kind. Infections by other fungi, including Saccharomyces, Aspergillus and Cryptococcus, are also called fungemia. It is most commonly seen in immunosuppressed or immunocompromised patients with severe neutropenia, cancer patients, or in patients with intravenous catheters. It has been suggested that otherwise immunocompetent patients taking infliximab may also be at a higher risk for fungemia.

Lacticaseibacillus rhamnosus is a bacterium that originally was considered to be a subspecies of L. casei, but genetic research found it to be a separate species in the L. casei clade, which also includes L. paracasei and L. zeae. It is a short Gram-positive homofermentative facultative anaerobic non-spore-forming rod that often appears in chains. Some strains of L. rhamnosus bacteria are being used as probiotics, and are particularly useful in treating infections of the female urogenital tract, most particularly very difficult to treat cases of bacterial vaginosis. The species Lacticaseibacillus rhamnosus and Limosilactobacillus reuteri are commonly found in the healthy female genito-urinary tract and are helpful to regain control of dysbiotic bacterial overgrowth during an active infection. L. rhamnosus sometimes is used in dairy products such as fermented milk and as non-starter-lactic acid bacterium (NSLAB) in long-ripened cheese. While frequently considered a beneficial organism, L. rhamnosus may not be as beneficial to certain subsets of the population; in rare circumstances, especially those primarily involving weakened immune system or infants, it may cause endocarditis. Despite the rare infections caused by L. rhamnosus, the species is included in the list of bacterial species with qualified presumed safety (QPS) status of the European Food Safety Agency.

Fecal microbiota transplant (FMT), also known as a stool transplant, is the process of transferring fecal bacteria and other microbes from a healthy individual into another individual. FMT is an effective treatment for Clostridioides difficile infection (CDI). For recurrent CDI, FMT is more effective than vancomycin alone, and may improve the outcome after the first index infection.

Rifaximin, is a non-absorbable, broad spectrum antibiotic mainly used to treat travelers' diarrhea. It is based on the rifamycin antibiotics family. Since its approval in Italy in 1987, it has been licensed in over more than 30 countries for the treatment of a variety of gastrointestinal diseases like irritable bowel syndrome, and hepatic encephalopathy. It acts by inhibiting RNA synthesis in susceptible bacteria by binding to the RNA polymerase enzyme. This binding blocks translocation, which stops transcription. It is marketed under the brand name Xifaxan by Salix Pharmaceuticals.
Antibiotic-associated diarrhea (AAD) results from an imbalance in the colonic microbiota caused by antibiotics. Microbiotal alteration changes carbohydrate metabolism with decreased short-chain fatty acid absorption and an osmotic diarrhea as a result. Another consequence of antibiotic therapy leading to diarrhea is overgrowth of potentially pathogenic organisms such as Clostridium difficile. It is defined as frequent loose and watery stools with no other complications.
Dysbiosis is characterized by a disruption to the microbiome resulting in an imbalance in the microbiota, changes in their functional composition and metabolic activities, or a shift in their local distribution. For example, a part of the human microbiota such as the skin flora, gut flora, or vaginal flora, can become deranged, with normally dominating species underrepresented and normally outcompeted or contained species increasing to fill the void. Dysbiosis is most commonly reported as a condition in the gastrointestinal tract.
Anti-Saccharomyces cerevisiae antibodies (ASCAs) are antibodies against antigens presented by the cell wall of the yeast Saccharomyces cerevisiae. These antibodies are directed against oligomannose sequences α-1,3 Man n. ASCAs and perinuclear antineutrophil cytoplasmic antibodies (pANCAs) are the two most useful and often discriminating biomarkers for colitis. ASCA tends to recognize Crohn's disease more frequently, whereas pANCA tend to recognize ulcerative colitis.
Helicobacter pylori eradication protocols is a standard name for all treatment protocols for peptic ulcers and gastritis in the presence of Helicobacter pylori infection. The primary goal of the treatment is not only temporary relief of symptoms but also total elimination of H. pylori infection. Patients with active duodenal or gastric ulcers and those with a prior ulcer history should be tested for H. pylori. Appropriate therapy should be given for eradication. Patients with MALT lymphoma should also be tested and treated for H. pylori since eradication of this infection can induce remission in many patients when the tumor is limited to the stomach. Several consensus conferences, including the Maastricht Consensus Report, recommend testing and treating several other groups of patients but there is limited evidence of benefit. This includes patients diagnosed with gastric adenocarcinoma, patients found to have atrophic gastritis or intestinal metaplasia, as well as first-degree relatives of patients with gastric adenocarcinoma since the relatives themselves are at increased risk of gastric cancer partly due to the intrafamilial transmission of H. pylori. To date, it remains controversial whether to test and treat all patients with functional dyspepsia, gastroesophageal reflux disease, or other non-GI disorders as well as asymptomatic individuals.

Clostridium butyricum is a strictly anaerobic endospore-forming Gram-positive butyric acid–producing bacillus subsisting by means of fermentation using an intracellularly accumulated amylopectin-like α-polyglucan (granulose) as a substrate. It is uncommonly reported as a human pathogen and is widely used as a probiotic in Asia. C. butyricum is a soil inhabitant in various parts of the world, has been cultured from the stool of healthy children and adults, and is common in soured milk and cheeses. The connection with dairy products is shown by the name, the butyr- in butyricum reflects the relevance of butyric acid in the bacteria's metabolism and the connection with Latin butyrum and Greek βούτυρον, with word roots pertaining to butter and cheese.

Fidaxomicin, sold under the brand name Dificid among others, is the first member of a class of narrow spectrum macrocyclic antibiotic drugs called tiacumicins. It is a fermentation product obtained from the actinomycete Dactylosporangium aurantiacum subspecies hamdenesis. Fidaxomicin is minimally absorbed into the bloodstream when taken orally, is bactericidal, and selectively eradicates pathogenic Clostridioides difficile with relatively little disruption to the multiple species of bacteria that make up the normal, healthy intestinal microbiota. The maintenance of normal physiological conditions in the colon may reduce the probability of recurrence of Clostridioides difficile infection.

Pediococcus acidilactici is a species of Gram-positive cocci that is often found in pairs or tetrads. P. acidilactici is a homofermentative bacterium that can grow in a wide range of pH, temperature, and osmotic pressure, therefore being able to colonize the digestive tract. It has emerged as a potential probiotic that has shown promising results in animal and human experiments, though some of the results are limited. They are commonly found in fermented vegetables, fermented dairy products, and meat.
Probiotics are live microorganisms promoted with claims that they provide health benefits when consumed, generally by improving or restoring the gut flora. Probiotics are considered generally safe to consume, but may cause bacteria-host interactions and unwanted side effects in rare cases. There is little evidence that probiotics bring the health benefits claimed for them.
Bacteriotherapy is the purposeful use of bacteria or their products in treating an illness. Forms of bacteriotherapy include the use of probiotics, microorganisms that provide health benefits when consumed; fecal matter transplants (FMT) /intestinal microbiota transplant (IMT), the transfer of gut microorganisms from the fecal matter of healthy donors to recipient patients to restore microbiota; or synbiotics which combine prebiotics, indigestible ingredients that promote growth of beneficial microorganisms, and probiotics. Through these methods, the gut microbiota, the community of 300-500 microorganism species that live in the digestive tract of animals aiding in digestion, energy storage, immune function and protection against pathogens, can be recolonized with favorable bacteria, which in turn has therapeutic effects.

Clostridioides difficile is a bacterium known for causing serious diarrheal infections, and may also cause colon cancer. It is known also as C. difficile, or C. diff, and is a Gram-positive species of spore-forming bacteria. Clostridioides spp. are anaerobic, motile bacteria, ubiquitous in nature and especially prevalent in soil. Its vegetative cells are rod-shaped, pleomorphic, and occur in pairs or short chains. Under the microscope, they appear as long, irregular cells with a bulge at their terminal ends. Under Gram staining, C. difficile cells are Gram-positive and show optimum growth on blood agar at human body temperatures in the absence of oxygen. C. difficile is catalase- and superoxide dismutase-negative, and produces up to three types of toxins: enterotoxin A, cytotoxin B and Clostridioides difficile transferase. Under stress conditions, the bacteria produce spores that are able to tolerate extreme conditions that the active bacteria cannot tolerate.
Henri Boulard was a French microbiologist who discovered the yeast Saccharomyces boulardii in 1923. He noticed people chewing on the skins of lychees and mangosteens to treat diarrhea during a cholera epidemic. He isolated and identified this strain of yeast, a probiotic. He sold his patented strain of saccharomyces boulardii to Biocodex, a French pharmaceutical company in the 1950s. This probiotic is used to improve gut health and treat diarrhea and is available in over 100 countries as Florastor.











