Related Research Articles
Peptic ulcer disease is a break in the inner lining of the stomach, the first part of the small intestine, or sometimes the lower esophagus. An ulcer in the stomach is called a gastric ulcer, while one in the first part of the intestines is a duodenal ulcer. The most common symptoms of a duodenal ulcer are waking at night with upper abdominal pain, and upper abdominal pain that improves with eating. With a gastric ulcer, the pain may worsen with eating. The pain is often described as a burning or dull ache. Other symptoms include belching, vomiting, weight loss, or poor appetite. About a third of older people with peptic ulcers have no symptoms. Complications may include bleeding, perforation, and blockage of the stomach. Bleeding occurs in as many as 15% of cases.
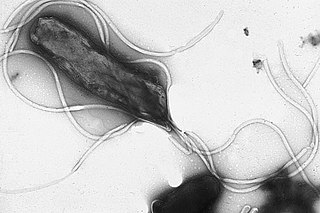
Helicobacter pylori, previously known as Campylobacter pylori, is a gram-negative, flagellated, helical bacterium. Mutants can have a rod or curved rod shape, and these are less effective. Its helical body is thought to have evolved in order to penetrate the mucous lining of the stomach, helped by its flagella, and thereby establish infection. The bacterium was first identified as the causal agent of gastric ulcers in 1983 by the Australian doctors Barry Marshall and Robin Warren.

Pantoprazole, sold under the brand name Protonix, among others, is a proton pump inhibitor used for the treatment of stomach ulcers, short-term treatment of erosive esophagitis due to gastroesophageal reflux disease (GERD), maintenance of healing of erosive esophagitis, and pathological hypersecretory conditions including Zollinger–Ellison syndrome. It may also be used along with other medications to eliminate Helicobacter pylori. Effectiveness is similar to other proton pump inhibitors (PPIs). It is available by mouth and by injection into a vein.
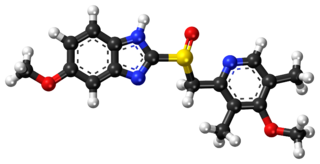
Esomeprazole, sold under the brand name Nexium [or Neksium] among others, is a medication which reduces stomach acid. It is used to treat gastroesophageal reflux disease, peptic ulcer disease, and Zollinger–Ellison syndrome. Its effectiveness is similar to that of other proton pump inhibitors (PPIs). It is taken by mouth or injection into a vein.

Gastritis is inflammation of the lining of the stomach. It may occur as a short episode or may be of a long duration. There may be no symptoms but, when symptoms are present, the most common is upper abdominal pain. Other possible symptoms include nausea and vomiting, bloating, loss of appetite and heartburn. Complications may include stomach bleeding, stomach ulcers, and stomach tumors. When due to autoimmune problems, low red blood cells due to not enough vitamin B12 may occur, a condition known as pernicious anemia.
Achlorhydria and hypochlorhydria refer to states where the production of hydrochloric acid in gastric secretions of the stomach and other digestive organs is absent or low, respectively. It is associated with various other medical problems.

This is a timeline of the events relating to the discovery that peptic ulcer disease and some cancers are caused by H. pylori. In 2005, Barry Marshall and Robin Warren were awarded the Nobel Prize in Physiology or Medicine for their discovery that peptic ulcer disease (PUD) was primarily caused by Helicobacter pylori, a bacterium with affinity for acidic environments, such as the stomach. As a result, PUD that is associated with H. pylori is currently treated with antibiotics used to eradicate the infection. For decades prior to their discovery, it was widely believed that PUD was caused by excess acid in the stomach. During this time, acid control was the primary method of treatment for PUD, to only partial success. Among other effects, it is now known that acid suppression alters the stomach milieu to make it less amenable to H. pylori infection.
Saccharomyces boulardii is a tropical yeast first isolated from lychee and mangosteen fruit peel in 1923 by French scientist Henri Boulard. Although early reports claimed distinct taxonomic, metabolic, and genetic properties, S. boulardii is genetically a grouping of S. cerevisiae strains, sharing >99% genomic relatedness, giving the synonym S. cerevisiae var. boulardii.
Helicobacter felis is a bacterial species in the Helicobacteraceae family, Campylobacterales order, Helicobacter genus. This bacterium is Gram-negative, microaerophilic, urease-positive, and spiral-shaped. Its type strain is CS1T. It can be pathogenic.
Helicobacter salomonis is a species within the Helicobacter genus of Gram-negative bacteria. Helicobacter pylori is by far the best known Helicobacter species primarily because humans infected with it may develop gastrointestinal tract diseases such as stomach inflammation, stomach ulcers, duodenal ulcers, stomach cancers of the nonlymphoma type, and various subtypes of extranodal marginal zone lymphomass, e.g. those of the stomach, small intestines, large intestines, and rectumn. H. pylori is also associated with the development of bile duct cancer and has been associated with a wide range of other diseases, although its role in the development of many of these other diseases requires further study. Humans infected with H. salomonis may develop some of the same gastrointestinal diseases viz., stomach inflammation, stomach ulcers, duodenal ulcers, stomach cancers that are not lymphomas, and extranodal marginal B cell lymphomas of the stomach. Other non-H. pylori Helicobacter species that are known to be associated with these gastrointestinal diseases are Helicobacter bizzozeronii, Helicobacter suis, Helicobacter felis, and Helicobacter heilmannii s.s. Because of their disease associations, these four Helicobacter species plus H. salomonis are often group together and termed Helicobacter heilmannii sensu lato.
The drug combination bismuth subcitrate/metronidazole/tetracycline is used for the treatment of peptic ulcer with an infection by the bacterium Helicobacter pylori. It is taken by mouth.

Vonoprazan, sold under the brand name Voquezna among others, is a first-in-class potassium-competitive acid blocker medication. Vonoprazan is used in form of the fumarate for the treatment of gastroduodenal ulcer and reflux esophagitis, and can be combined with antibiotics for the eradication of Helicobacter pylori. It is a potassium-competitive acid blocker.
Bismuth subcitrate potassium is a bismuth salt used in combination with antibiotics and a proton pump inhibitor for the treatment of Helicobacter pylori infections.
Helicobacter heilmannii sensu lato refers to a group of bacterial species within the Helicobacter genus. The Helicobacter genus consists of at least 40 species of spiral-shaped flagellated, Gram-negative bacteria of which the by far most prominent and well-known species is Helicobacter pylori. H. pylori is associated with the development of gastrointestinal tract diseases such as stomach inflammation, stomach ulcers, duodenal ulcers, stomach cancers that are not lymphomas, and various subtypes of extranodal marginal zone lymphomas, e.g. those of the stomach, small intestines, large intestines, and rectum. H. pylori has also been associated with the development of bile duct cancer and has been associated with a wide range of other diseases although its role in the development of many of these other diseases requires further study.
Helicobacter bizzozeronii is a species within the Helicobacter genus of Gram-negative bacteria. Helicobacter pylori is by far the best known Helicobacter species, primarily because humans infected with it may develop gastrointestinal tract diseases such as stomach inflammation, stomach ulcers, duodenal ulcers, stomach cancers of the nonlymphoma type, and various subtypes of extranodal marginal zone lymphomass, e.g. those of the stomach, small intestines, large intestines, and rectumn. H. pylori is also associated with the development of bile duct cancer and has been associated with a wide range of other diseases although its role in the development of many of these other diseases requires further study. Humans infected with H. bizzozeronii are prone to develop some of the same gastrointestinal diseases viz., stomach inflammation, stomach ulcers, duodenal ulcers, stomach cancers that are not lymphomas, and extranodal marginal B cell lymphomas of the stomach. Other non-H. pylori Helicobacter species that are known to be associated with these gastrointestinal diseases are Helicobacter felis, Helicobacter salomonis, Helicobacter suis, and Helicobacter heilmannii s.s. Because of their disease associations, these four Helicobacter species plus H. bizzozeronii are often grouped together and termed Helicobacter heilmannii sensu lato.
Helicobacter suis is a species within the Helicobacter genus of Gram-negative bacteria. Helicobacter pylori is by far the best known Helicobacter species, primarily because humans infected with it may develop gastrointestinal tract diseases such as stomach inflammation, stomach ulcers, duodenal ulcers, stomach cancers of the nonlymphoma type, and various subtypes of extranodal marginal zone lymphomass, e.g. those of the stomach, small intestines, large intestines, and rectumn. H. pylori is also associated with the development of bile duct cancer and has been associated with a wide range of other diseases although its role in the development of many of these other diseases requires further study. Humans infected with H. suis may develop some of the same gastrointestinal diseases - stomach inflammation, stomach ulcers, duodenal ulcers, stomach cancers that are not lymphomas, and extranodal marginal B cell lymphomas of the stomach. Other non-H. pylori Helicobacter species that are known to be associated with these gastrointestinal diseases are Helicobacter bizzozeronii, Helicobacter salomonis, Helicobacter felis, and Helicobacter heilmannii s.s. Because of their disease associations, these four Helicobacter species plus H. suis are often group together and termed Helicobacter heilmannii sensu lato.
Helicobacter heilmannii s.s. is a species within the Helicobacter genus of Gram negative bacteria. Helicobacter pylori is by far the best known Helicobacter species primarily because humans infected with it may develop gastrointestinal tract diseases such as stomach inflammation, stomach ulcers, duodenal ulcers, stomach cancers of the non-lymphoma type, and various subtypes of extranodal marginal zone lymphomass, e.g. those of the stomach, small intestines, large intestines, and rectum. H. pylori is also associated with the development of bile duct cancer and has been associated with a wide range of other diseases although its role in the development of many of these other diseases requires further study. Humans infected with H. heilmannii s.s. may develop some of the same gastrointestinal diseases viz., stomach inflammation, stomach ulcers, duodenal ulcers, stomach cancers that are not lymphomas, and extranodal marginal B cell lymphomas of the stomach. Other non-H. pylori Helicobacter species that are known to be associated with these gastrointestinal diseases are Helicobacter bizzozeronii, Helicobacter suis, Helicobacter felis, and Helicobacter salomonis. Because of their disease associations, these four Helicobacter species plus H. heilmannii s.s. are often group together and termed Helicobacter heilmannii sensu lato.
Ranitidine bismuth citrate - drug, which has antisecretory and bactericidal action.
Vonoprazan/amoxicillin/clarithromycin, sold under the brand name Vonosap among others, is a co-packaged medication used for the treatment of Helicobacter pylori infection. It contains vonoprazan, a potassium-competitive acid blocker; amoxicillin, a beta-lactam antibiotic; and clarithromycin, a macrolide antibiotic.

Vonoprazan/amoxicillin, sold under the brand name Voquezna Dual Pak among others, is a co-packaged medication used for the treatment of Helicobacter pylori infection. It contains vonoprazan, a potassium-competitive acid blocker and amoxicillin, a beta-lactam antibiotic. It is taken by mouth.
References
- ↑ Chan FK, To KF, Wu JC, Yung MY, Leung WK, Kwok T, Hui Y, Chan HL, Chan CS, Hui E, Woo J, Sung JJ (5 January 2002). "Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: a randomised trial". Lancet. 359 (9300): 9–13. doi:10.1016/s0140-6736(02)07272-0. PMID 11809180. S2CID 26114789.
- ↑ Sonnenberg A (June 2007). "Time trends of ulcer mortality in Europe". Gastroenterology. 132 (7): 2320–7. doi: 10.1053/j.gastro.2007.03.108 . PMID 17570207.
- 1 2 3 Gatta L, Vakil N, Vaira D, Scarpignato C (7 August 2013). "Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy". BMJ (Clinical Research Ed.). 347: f4587. doi:10.1136/bmj.f4587. PMC 3736972 . PMID 23926315.
- 1 2 Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ (5 April 2012). "Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report". Gut. 61 (5): 646–664. doi: 10.1136/gutjnl-2012-302084 . hdl: 1765/64813 . PMID 22491499.
- ↑ Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia-Abadia E, Vinagre-Rodriguez G, Martinez-Alcala C, Hernandez-Alonso M, Miranda A, Iovene MR, Pazos-Pacheco C, Gisbert JP (July 2013). "Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance". Gastroenterology. 145 (1): 121–128.e1. doi:10.1053/j.gastro.2013.03.050. PMID 23562754.
- ↑ Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ (June 2007). "Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report". Gut. 56 (6): 772–81. doi:10.1136/gut.2006.101634. PMC 1954853 . PMID 17170018.
- ↑ Wang H, Kong QZ, Li YY, Yang XY, Zuo XL (March 2024). "High-dose dual therapy versus bismuth-containing quadruple therapy for the eradication of Helicobacter pylori: A systematic review and meta-analysis". J Dig Dis. 25 (3): 163–175. doi:10.1111/1751-2980.13263. PMID 38577962.
- ↑ Urgesi R, Cianci R, Riccioni ME (2012). "Update on triple therapy for eradication of Helicobacter pylori: current status of the art". Clinical and Experimental Gastroenterology. 5: 151–157. doi: 10.2147/CEG.S25416 . PMC 3449761 . PMID 23028235.
- ↑ Rimbara E, Fischbach LA, Graham DY (2011). "Optimal therapy for Helicobacter pylori infections". Nat. Rev. Gastroenterol. Hepatol. 8 (2): 79–88. doi:10.1038/nrgastro.2010.210. PMID 21293508. S2CID 23529476.
- ↑ Zhou BG, Jiang X, Ding YB, She Q, Li YY (2024). "Vonoprazan-amoxicillin dual therapy versus bismuth-containing quadruple therapy for Helicobacter pylori eradication: A systematic review and meta-analysis". Helicobacter. 29 (1): e13040. doi:10.1111/hel.13040. PMID 37983865. S2CID 265308205.
- ↑ Ren Q, Yan X, Zhou Y, Li WX (7 February 2016). "Periodontal therapy as adjunctive treatment for gastric Helicobacter pylori infection". The Cochrane Database of Systematic Reviews. 2016 (2): CD009477. doi:10.1002/14651858.CD009477.pub2. PMC 8255095 . PMID 26852297.
- ↑ Szajewska H, Horvath A, Piwowarczyk A (2010). "Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment". Aliment Pharmacol Ther. 32 (9): 1069–1079. doi: 10.1111/j.1365-2036.2010.04457.x . PMID 21039671. Archived from the original on 2019-09-02. Retrieved 2019-09-02.
- ↑ B Y, H A (2010). "Efficacy of probiotics in Helicobacter pylori eradication therapy". Turk J Gastroenterol. 21 (3): 212–217. doi:10.4318/tjg.2010.0090. PMID 20931422.
- ↑ Borody TJ, P. Cole, S. Noonan, A. Morgan, J. Lenne, L. Hyland, S. Brandl, E. G. Borody, L. L. George (October 16, 1989). "Recurrence of duodenal ulcer and Campylobacter pylori infection after eradication". Medical Journal of Australia. 151 (8): 431–435. doi:10.5694/j.1326-5377.1989.tb101251.x. PMID 2687668. S2CID 26066525.
- 1 2 Mirbagheri SA, Mehrdad Hasibi, Mehdi Abouzari, Armin Rashidi (August 14, 2006). "Triple, standard quadruple and ampicillin–sulbactam-based quadruple therapies for H pylori eradication: A comparative three-armed randomized clinical trial". World Journal of Gastroenterology. 12 (30): 4888–4891. doi: 10.3748/wjg.v12.i30.4888 . PMC 4087627 . PMID 16937475.
- ↑ Cammarota G, Branca G, Ardito F, Sanguinetti M, Ianiro G, Cianci R, Torelli R, Masala G, Gasbarrini A, Fadda G, Landolfi R, Gasbarrini G (2010). "Biofilm Demolition and Antibiotic Treatment to Eradicate Resistant Helicobacter pylori: A Clinical Trial". Clinical Gastroenterology and Hepatology. 8 (9): 817–820.e3. doi:10.1016/j.cgh.2010.05.006. PMID 20478402.
- ↑ Kanu JE, Soldera J (March 2024). "Treatment of Helicobacter pylori with potassium competitive acid blockers: A systematic review and meta-analysis". World J Gastroenterol. 30 (9): 1213–1223. doi: 10.3748/wjg.v30.i9.1213 . PMC 10989498 . PMID 38577188.