Related Research Articles

Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity (electrowinning).

Peptic ulcer disease (PUD) is a break in the inner lining of the stomach, the first part of the small intestine, or sometimes the lower esophagus. An ulcer in the stomach is called a gastric ulcer, while one in the first part of the intestines is a duodenal ulcer. The most common symptoms of a duodenal ulcer are waking at night with upper abdominal pain, and upper abdominal pain that improves with eating. With a gastric ulcer, the pain may worsen with eating. The pain is often described as a burning or dull ache. Other symptoms include belching, vomiting, weight loss, or poor appetite. About a third of older people have no symptoms. Complications may include bleeding, perforation, and blockage of the stomach. Bleeding occurs in as many as 15% of cases.

Iron deficiency, or sideropenia, is the state in which a body lacks enough iron to supply its needs. Iron is present in all cells in the human body and has several vital functions, such as carrying oxygen to the tissues from the lungs as a key component of the hemoglobin protein, acting as a transport medium for electrons within the cells in the form of cytochromes, and facilitating oxygen enzyme reactions in various tissues. Too little iron can interfere with these vital functions and lead to morbidity and death.

A mouth ulcer (aphtha) is an ulcer that occurs on the mucous membrane of the oral cavity. Mouth ulcers are very common, occurring in association with many diseases and by many different mechanisms, but usually there is no serious underlying cause. Rarely, a mouth ulcer that does not heal may be a sign of oral cancer. These ulcers may form individually or multiple ulcers may appear at once. Once formed, an ulcer may be maintained by inflammation and/or secondary infection.

Iron-deficiency anemia is anemia caused by a lack of iron. Anemia is defined as a decrease in the number of red blood cells or the amount of hemoglobin in the blood. When onset is slow, symptoms are often vague such as feeling tired, weak, short of breath, or having decreased ability to exercise. Anemia that comes on quickly often has more severe symptoms, including confusion, feeling like one is going to pass out or increased thirst. Anemia is typically significant before a person becomes noticeably pale. Children with iron deficiency anemia may have problems with growth and development. There may be additional symptoms depending on the underlying cause.
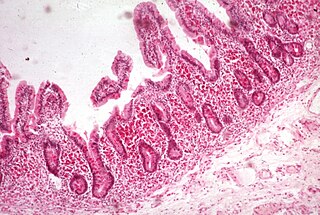
Malabsorption is a state arising from abnormality in absorption of food nutrients across the gastrointestinal (GI) tract. Impairment can be of single or multiple nutrients depending on the abnormality. This may lead to malnutrition and a variety of anaemias.

Aphthous stomatitis, or recurrent aphthous stomatitis (RAS), is a common condition characterized by the repeated formation of benign and non-contagious mouth ulcers (aphthae) in otherwise healthy individuals. The informal term canker sore is also used, mainly in North America, although it may also refer to other types of mouth ulcers. The cause is not completely understood but involves a T cell-mediated immune response triggered by a variety of factors which may include nutritional deficiencies, local trauma, stress, hormonal influences, allergies, genetic predisposition, certain foods, dehydration, some food additives, or some hygienic chemical additives like SDS.

Anserine (β-alanyl-3-methylhistidine) is a dipeptide containing β-alanine and 3-methylhistidine. Anserine is a derivative of carnosine, which has been methylated. Both anserine and carnosine are capable of chelating copper. Due to its methylation, anserine is more stable in serum and resistant to degradation than carnosine. Anserine can be found in the skeletal muscle and brain of mammals and birds. It can also be found in high levels in the human kidneys. The pKa of the imidazole ring of histidine, when contained in anserine, is 7.04, making it an effective buffer at physiologic pH.

Sucralfate, sold under various brand names, is a medication used to treat stomach ulcers, gastroesophageal reflux disease (GERD), radiation proctitis, and stomach inflammation and to prevent stress ulcers. Its usefulness in people infected by H. pylori is limited. It is used by mouth and rectally.
Zinc toxicity is a medical condition involving an overdose on, or toxic overexposure to, zinc. Such toxicity levels have been seen to occur at ingestion of greater than 50 mg of zinc. Excessive absorption of zinc can suppress copper and iron absorption. The free zinc ion in solution is highly toxic to bacteria, plants, invertebrates, and even vertebrate fish. Zinc is an essential trace metal with very low toxicity in humans.
Copper deficiency, or hypocupremia, is defined either as insufficient copper to meet the needs of the body, or as a serum copper level below the normal range. Symptoms may include fatigue, decreased red blood cells, early greying of the hair, and neurological problems presenting as numbness, tingling, muscle weakness, and ataxia. The neurodegenerative syndrome of copper deficiency has been recognized for some time in ruminant animals, in which it is commonly known as "swayback". Copper deficiency can manifest in parallel with vitamin B12 and other nutritional deficiencies.

Amlexanox is an anti-inflammatory antiallergic immunomodulator used to treat recurrent aphthous ulcers, and several inflammatory conditions. This drug has been discontinued in the U.S.
Zinc deficiency is defined either as insufficient zinc to meet the needs of the body, or as a serum zinc level below the normal range. However, since a decrease in the serum concentration is only detectable after long-term or severe depletion, serum zinc is not a reliable biomarker for zinc status. Common symptoms include increased rates of diarrhea. Zinc deficiency affects the skin and gastrointestinal tract; brain and central nervous system, immune, skeletal, and reproductive systems.

Troxipide is a drug used in the treatment of gastroesophageal reflux disease. Troxipide is a systemic non-antisecretory gastric cytoprotective agent with anti-ulcer, anti-inflammatory and mucus secreting properties irrespective of pH of stomach or duodenum. Troxipide is currently marketed in Japan (Aplace), China (Shuqi), South Korea (Defensa), and India (Troxip). It is used for the management of gastric ulcers, and amelioration of gastric mucosal lesions in acute gastritis and acute exacerbation of chronic gastritis.

Nutritional neuroscience is the scientific discipline that studies the effects various components of the diet such as minerals, vitamins, protein, carbohydrates, fats, dietary supplements, synthetic hormones, and food additives have on neurochemistry, neurobiology, behavior, and cognition.
Cytoprotection is a process by which chemical compounds provide protection to cells against harmful agents.
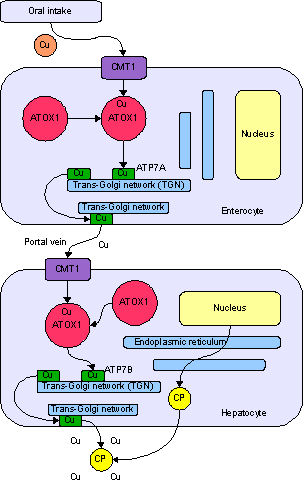
Copper is an essential trace element that is vital to the health of all living things. In humans, copper is essential to the proper functioning of organs and metabolic processes. The human body has complex homeostatic mechanisms which attempt to ensure a constant supply of available copper, while eliminating excess copper whenever this occurs. However, like all essential elements and nutrients, too much or too little nutritional ingestion of copper can result in a corresponding condition of copper excess or deficiency in the body, each of which has its own unique set of adverse health effects.
Treatment of the inherited blood disorder thalassemia depends upon the level of severity. For mild forms of the condition, advice and counseling are often all that are necessary. For more severe forms, treatment may consist in blood transfusion; chelation therapy to reverse iron overload, using drugs such as deferoxamine, deferiprone, or deferasirox; medication with the antioxidant indicaxanthin to prevent the breakdown of hemoglobin; or a bone marrow transplant using material from a compatible donor, or from the patient's mother. Removal of the spleen (splenectomy) could theoretically help to reduce the need for blood transfusions in people with thalassaemia major or intermedia but there is currently no reliable evidence from clinical trials about its effects. Population screening has had some success as a preventive measure.

Barbara Jean Francis Apgar is an American biochemist. She worked on important research on ribonucleic acids (RNA), and on zinc deficiency as a risk factor in reproduction. She won the Federal Woman's Award in 1970, and the Arthur S. Flemming Award in 1973.

Zinc is an essential trace element for humans and other animals, for plants and for microorganisms. Zinc is required for the function of over 300 enzymes and 1000 transcription factors, and is stored and transferred in metallothioneins. It is the second most abundant trace metal in humans after iron and it is the only metal which appears in all enzyme classes.
References
- ↑ Yamaguchi M (1995). "beta-Alanyl-L-histidinato zinc and bone resorption". General Pharmacology. 26 (6): 1179–83. doi:10.1016/0306-3623(95)00008-o. PMID 7590105.
beta-Alanyl-L-histidinato zinc (AHZ), in which zinc is chelated to beta-alanyl-L-histidine, is a new zinc compound.
- ↑ Yoshikawa T, Naito Y, Kondo M (1993). "Antioxidant therapy in digestive diseases". Journal of Nutritional Science and Vitaminology. 39 Suppl: S35–41. doi: 10.3177/jnsv.39.supplement_s35 . PMID 8164065.
Zinc-carnosine (Z-103), N-(3-aminopropionyl)-L-histidinato zinc, is a chelate compound consisting of zinc ion and L-carnosine
- 1 2 3 Takei M (2012). "[Development of polaprezinc research]". Yakugaku Zasshi (in Japanese). 132 (3): 271–7. doi: 10.1248/yakushi.132.271 . PMID 22382829.
Polaprezinc (Promac(®), Zeria Pharmaceutical Co., Ltd.), a chelate compound consisting of zinc and L-carnosine, is a zinc-related medicine approved for the first time in Japan, which has been clinically used to treat gastric ulcers. Its mechanism of action is believed to oxygen radical scavenging, anti-oxidation, and acceleration of wound healing.
- 1 2 Palileo C, Kaunitz JD (2011). "Gastrointestinal defense mechanisms". Current Opinion in Gastroenterology. 27 (6): 543–8. doi:10.1097/MOG.0b013e32834b3fcb. PMC 5667561 . PMID 21897225.
The mucosal protective drug polaprezinc exhibits ROS-quenching activities.
- 1 2 Dajani EZ, Klamut MJ (2000). "Novel therapeutic approaches to gastric and duodenal ulcers: an update". Expert Opinion on Investigational Drugs. 9 (7): 1537–44. doi:10.1517/13543784.9.7.1537. PMID 11060758. S2CID 28302630.
•Polaprezinc and nocloprost are also mucosal protective drugs, which are in clinical development.
•Its mechanism of action is not totally known, but it has been shown to stimulate mucus production and to maintain the integrity of the gastric mucosal barrier [51]. - 1 2 3 4 5 6 7 8 9 Matsukura T, Tanaka H (2000). "Applicability of zinc complex of L-carnosine for medical use". Biochemistry. 65 (7): 817–23. PMID 10951100 . Retrieved 2016-06-20.
- ↑ Matsukura, T; Takahashi, T; Nishimura, Y; Ohtani, T; Sawada, M; Shibata, K (November 1990). "Characterization of crystalline L-carnosine Zn(II) complex (Z-103), a novel anti-gastric ulcer agent: tautomeric change of imidazole moiety upon complexation". Chem Pharm Bull. 38 (11): 3140–6. doi: 10.1248/cpb.38.3140 . PMID 2085900.
- 1 2 3 4 Sakae K, Yanagisawa H (2014). "Oral treatment of pressure ulcers with polaprezinc (zinc L-carnosine complex): 8-week open-label trial". Biological Trace Element Research. 158 (3): 280–8. doi:10.1007/s12011-014-9943-5. PMID 24691900. S2CID 2116866.
•Patients with stage II-IV pressure ulcers for ≥ 8 weeks received 150 mg/day polaprezinc (containing 116 mg L-carnosine and 34 mg zinc) per os for a maximum of 8 weeks.
•Serum zinc levels increased significantly (P < 0.001), whereas serum copper levels (P= 0.001) and copper/zinc ratios (P < 0.001) decreased significantly. In one patient, preexisting copper deficiency deteriorated. - 1 2 Ooi TC, Chan KM, Sharif R (2017). "Antioxidant, Anti-inflammatory, and Genomic Stability Enhancement Effects of Zinc l-carnosine: A Potential Cancer Chemopreventive Agent?". Nutrition and Cancer. 69 (2): 201–210. doi:10.1080/01635581.2017.1265132. PMID 28094570. S2CID 40817163.
Zinc L-carnosine (ZnC), which is clinically used as gastric ulcer treatment in Japan, has been suggested to have the potential in preventing cancer development. Multiple studies have revealed that ZnC possesses potent antioxidant, antiinflammatory, and genomic stability enhancement effects.
- 1 2 3 4 5 6 7 8 9 10 11 Doi H, Kuribayashi K, Kijima T (August 2018). "Utility of polaprezinc in reducing toxicities during radiotherapy: a literature review". Future Oncology (London, England). 14 (19): 1977–1988. doi:10.2217/fon-2018-0021. PMID 30074413. S2CID 51906416.
- ↑ "Zeria Of Japan Obtains Korean Approval For Ulcer Drug Polaprezinc". Pink Sheet - Informa Pharma Intelligence. Retrieved 28 May 2020.
- ↑ "Lonza Announces New Dietary Supplement Ingredient PepZin GI™". New Hope Network. 18 September 2002. Retrieved 28 May 2020.
- ↑ "Therapeutic Goods (Permissible Ingredients) Determination (No.1) 2020 - Volume 5". Federal Register of Legislation. Australian Government. Retrieved 28 May 2020.
- ↑ "Chemical Substance - Polaprezinc". Natural Health Products Ingredients Database. Health Canada. 26 July 2004. Retrieved 28 May 2020.
- ↑ Samborski P, Grzymisławski M (2015). "The Role of HSP70 Heat Shock Proteins in the Pathogenesis and Treatment of Inflammatory Bowel Diseases". Advances in Clinical and Experimental Medicine. 24 (3): 525–30. doi: 10.17219/acem/44144 . PMID 26467144.
•In experimental studies, overexpression of HSP70 was found to prevent the development of inflammatory process in the large intestinal mucosa provoked by various damaging factors.
•There is also a potential for pharmacological stimulation of HSP70 expression, linked (for example) to geranylgeranylacetone, polaprezinc and mesalazine. - ↑ Yamaguchi M (2010). "Role of nutritional zinc in the prevention of osteoporosis". Molecular and Cellular Biochemistry. 338 (1–2): 241–54. doi:10.1007/s11010-009-0358-0. PMID 20035439. S2CID 35574730.
beta-Alanyl-L: -histidinato zinc (AHZ) is a zinc compound, in which zinc is chelated to beta-alanyl-L: -histidine. The stimulatory effect of AHZ on bone formation is more intensive than that of zinc sulfate. Zinc acexamate has also been shown to have a potent-anabolic effect on bone. The oral administration of AHZ or zinc acexamate has the restorative effect on bone loss under various pathophysiologic conditions including aging, skeletal unloading, aluminum bone toxicity, calcium- and vitamin D-deficiency, adjuvant arthritis, estrogen deficiency, diabetes, and fracture healing.
- 1 2 Sakae K, Agata T, Kamide R, Yanagisawa H (2013). "Effects of L-carnosine and its zinc complex (Polaprezinc) on pressure ulcer healing". Nutrition in Clinical Practice. 28 (5): 609–16. doi:10.1177/0884533613493333. PMID 23835365.
Forty-two patients with stage II-IV pressure ulcers for 4 or more weeks were allocated to 1 of 3 groups in order of recruitment: the control group (n = 14) was untreated, the PLZ group (n = 10) orally received 150 mg/d PLZ (containing 116 mg CAR and 34 mg zinc), and the CAR group (n = 18) orally received 116 mg/d CAR.
- ↑ Institute of Medicine (US) Panel on Micronutrients (2001). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. ISBN 9780309072793. PMID 25057538 . Retrieved 2016-06-25.
A LOAEL of 60 mg/day was divided by a UF of 1.5 to derive a UL of 40 mg/day for total intake of zinc from food, water, and supplements.
- 1 2 Tan B, Luo HQ, Xu H, Lv NH, Shi RH, Luo HS, Li JS, Ren JL, Zou YY, Li YQ, Ji F, Fang JY, Qian JM (2017). "Polaprezinc Combined With Clarithromycin-Based Triple Therapy for Helicobacter Pylori-Associated Gastritis: A Prospective, Multicenter, Randomized Clinical Trial". PLOS ONE. 12 (4): e0175625. Bibcode:2017PLoSO..1275625T. doi: 10.1371/journal.pone.0175625 . PMC 5391070 . PMID 28407007.
- ↑ "インタビューフォーム". Zeria Medical Site. Zeria Pharmaceutical Co., Ltd. Retrieved 28 May 2020.
Accessible only to health professionals. Text in Japanese.
- ↑ "Pharmaceutical and Medical Devices Safety Information, No. 339, December 2016" (PDF). Pharmaceutical and Medical Devices Agency. PMDA, Government of Japan. Retrieved 28 May 2020.
- ↑ F. Aguilar, H. Autrup, S. Barlow, L. Castle, R. Crebelli, W. Dekant, K.-H. Engel, N. Gontard, D. Gott, S. Grilli, R. Gürtler, J.-C. Larsen, C. Leclercq, J.-C. Leblanc, F. X. Malcata, W. Mennes, M.-R. Milana, I. Pratt, I. Rietjens, P. Tobback, F. Toldrá (2008). "Opinion on certain bisglycinates as sources of copper, zinc, calcium, magnesium and glycinate nicotinate as source of chromium in foods intended for the general population (including food supplements) and foods for particular nutritional uses - Scientific Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food". EFSA Journal. 6 (6): 718. doi: 10.2903/j.efsa.2008.718 .
{{cite journal}}: CS1 maint: uses authors parameter (link)