Related Research Articles

Technetium is a chemical element with the symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, the most common source, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.

Iodine heptafluoride, also known as iodine(VII) fluoride or iodine fluoride, is an interhalogen compound with the chemical formula IF7. It has an unusual pentagonal bipyramidal structure, as predicted by VSEPR theory. The molecule can undergo a pseudorotational rearrangement called the Bartell mechanism, which is like the Berry mechanism but for a heptacoordinated system. It forms colourless crystals, which melt at 4.5 °C: the liquid range is extremely narrow, with the boiling point at 4.77 °C. The dense vapor has a mouldy, acrid odour. The molecule has D5h symmetry.
Fluoride volatility is the tendency of highly fluorinated molecules to vaporize at comparatively low temperatures. Heptafluorides, hexafluorides and pentafluorides have much lower boiling points than the lower-valence fluorides. Most difluorides and trifluorides have high boiling points, while most tetrafluorides and monofluorides fall in between. The term "fluoride volatility" is jargon used particularly in the context of separation of radionuclides.

Sodium pertechnetate is the inorganic compound with the formula NaTcO4. This colourless salt contains the pertechnetate anion, TcO−
4. The radioactive 99m
Tc
O−
4 anion is an important radiopharmaceutical for diagnostic use. The advantages to 99m
Tc
include its short half-life of 6 hours and the low radiation exposure to the patient, which allow a patient to be injected with activities of more than 30 millicuries. Na[99m
Tc
O
4] is a precursor to a variety of derivatives that are used to image different parts of the body.

Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.

Pertechnetic acid (HTcO4) is a compound of technetium that is produced by reacting technetium(VII) oxide (Tc2O7) with water or strong oxidizing acids, such as nitric acid, concentrated sulfuric acid or aqua regia. The dark red hygroscopic substance is a strong acid, with a pKa of 0.32, as such it exists almost entirely as the pertechnetate ion in aqueous solution. The red color in solution is thought to be due to the formation of the polyoxometallate Tc20O4−68.

Rhenium heptafluoride is the compound with the formula ReF7. It is a yellow low melting solid and is the only thermally stable metal heptafluoride. It has a distorted pentagonal bipyramidal structure similar to IF7, which was confirmed by neutron diffraction at 1.5 K. The structure is non-rigid, as evidenced by electron diffraction studies.

Technetium(IV) chloride is the inorganic compound with the formula TcCl4. It was discovered in 1957 as the first binary halide of technetium. It is the highest oxidation binary chloride of technetium that has been isolated as a solid. It is volatile at elevated temperatures and its volatility has been used for separating technetium from other metal chlorides. Colloidal solutions of technetium(IV) chloride are oxidized to form Tc(VII) ions when exposed to gamma rays.

The tetrafluoroammonium cation is a positively charged polyatomic ion with chemical formula NF+
4. It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine. Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane CF
4, trifluoramine oxide ONF
3 and the tetrafluoroborate BF−
4 anion.
Iodine monofluoride is an interhalogen compound of iodine and fluorine with formula IF. It is a chocolate-brown solid that decomposes at 0 °C, disproportionating to elemental iodine and iodine pentafluoride:

Heptafluoride typically refers to compounds with the formula RnMxF7y− or RnMxF7y+, where n, x, and y are independent variables and R any substituent.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
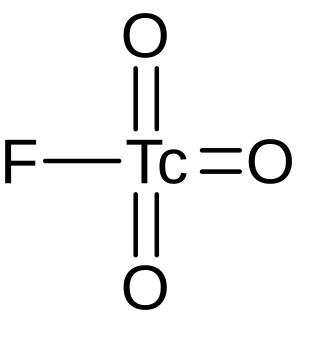
Pertechnetyl fluoride is an inorganic compound, a salt of technetium and hydrofluoric acid with the chemical formula TcO
3F. The compound was originally synthesized by H. Selig and G. Malm in 1963.
Osmium(IV) fluoride is an inorganic chemical compound of osmium metal and fluorine with the chemical formula OsF4.
Rhenium pentafluoride is a binary inorganic compound of rhenium and fluorine with the chemical formula ReF5. This is a salt of rhenium and hydrofluoric acid.
Osmium heptafluoride is an inorganic chemical compound of osmium metal and fluorine with the chemical formula OsF
7.
References
- ↑ "WebElements Periodic Table » Technetium » technetium pentafluoride". webelements.com. Retrieved 19 April 2023.
- ↑ Gutmann, Viktor (2 December 2012). Halogen Chemistry. Elsevier. p. 197. ISBN 978-0-323-14847-4 . Retrieved 19 April 2023.
- ↑ Schwochau, Klaus (21 November 2008). Technetium: Chemistry and Radiopharmaceutical Applications. John Wiley & Sons. p. 113. ISBN 978-3-527-61337-3 . Retrieved 19 April 2023.
- ↑ "Some physical properties of technetium pentafluoride". Journal of Inorganic and Nuclear Chemistry . 28: 231–232. 1 January 1976. doi:10.1016/0022-1902(76)80635-5. ISSN 0022-1902 . Retrieved 19 April 2023.
- ↑ Schwochau, Klaus (21 November 2008). Technetium: Chemistry and Radiopharmaceutical Applications. John Wiley & Sons. p. 114. ISBN 978-3-527-61337-3 . Retrieved 19 April 2023.
- ↑ Lide, David R. (29 June 2004). CRC Handbook of Chemistry and Physics, 85th Edition. CRC Press. p. 4-88. ISBN 978-0-8493-0485-9 . Retrieved 19 April 2023.
- ↑ Kemmitt, R. D. W.; Peacock, R. D. (26 January 2016). The Chemistry of Manganese, Technetium and Rhenium: Pergamon Texts in Inorganic Chemistry. Elsevier. p. 889. ISBN 978-1-4831-8762-4 . Retrieved 19 April 2023.