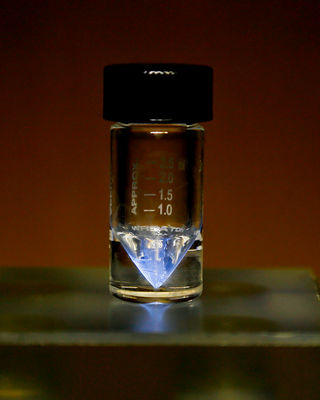
Actinium is a chemical element; it has symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name emanium; the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899 and called actinium. Actinium gave the name to the actinide series, a set of 15 elements between actinium and lawrencium in the periodic table. Together with polonium, radium, and radon, actinium was one of the first non-primordial radioactive elements to be isolated.

Americium is a synthetic chemical element; it has symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element europium and was thus named after the Americas by analogy.
The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.

Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.

Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor, and inorganic acids. It forms various chemical compounds, in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity, and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel.

Few compounds of californium have been made and studied. The only californium ion that is stable in aqueous solutions is the californium(III) cation. The other two oxidation states are IV (strong oxidizing agents) and II (strong reducing agents). The element forms a water-soluble chloride, nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate or hydroxide. If problems of availability of the element could be overcome, then CfBr2 and CfI2 would likely be stable.

Berkelium forms a number of chemical compounds, where it normally exists in an oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid. Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halogens, chalcogens and pnictogens to form various binary compounds. Berkelium can also form several organometallic compounds.
Actinium(III) oxide is a chemical compound containing the rare radioactive element actinium. It has the formula Ac2O3. It is similar to its corresponding lanthanum compound, lanthanum(III) oxide, and contains actinium in the oxidation state +3. Actinium oxide is not to be confused with Ac2O (acetic anhydride), where Ac is an abbreviation for acetyl instead of the symbol of the element actinium.

Lanthanum trifluoride is a refractory ionic compound of lanthanum and fluorine. The chemical formula is LaF
3.

Promethium(III) fluoride or promethium trifluoride is a salt of promethium and fluorine with the formula PmF3.
Neptunium(III) fluoride or neptunium trifluoride is a salt of neptunium and fluorine with the formula NpF3.

Neptunium(IV) fluoride or neptunium tetrafluoride is a inorganic compound with the formula NpF4. It is a green salt and is isostructural with UF4.
Neptunium(V) fluoride or neptunium pentafluoride is a chemical compound of neptunium and fluorine with the formula NpF5.
Curium compounds are compounds containing the element curium (Cm). Curium usually forms compounds in the +3 oxidation state, although compounds with curium in the +4, +5 and +6 oxidation states are also known.

Protactinium(V) fluoride is a fluoride of protactinium with the chemical formula PaF5.
Actinium compounds are compounds containing the element actinium (Ac). Due to actinium's intense radioactivity, only a limited number of actinium compounds are known. These include: AcF3, AcCl3, AcBr3, AcOF, AcOCl, AcOBr, Ac2S3, Ac2O3, AcPO4 and Ac(NO3)3. Except for AcPO4, they are all similar to the corresponding lanthanum compounds. They all contain actinium in the oxidation state +3. In particular, the lattice constants of the analogous lanthanum and actinium compounds differ by only a few percent.
Thorium oxyfluoride is an inorganic compound of thorium metal, fluorine, and oxygen with the chemical formula ThOF
2.
Actinium oxyfluoride is an inorganic compound, with the chemical formula AcOF. It is radioactive. It crystallises in a calcium fluoride structure. It can be obtained by reacting actinium fluoride with ammonia and water:
Einsteinium fluoride is a binary inorganic chemical compound of einsteinium and fluorine with the chemical formula EsF3.













