
Hereditary haemochromatosis type 1 is a genetic disorder characterized by excessive intestinal absorption of dietary iron, resulting in a pathological increase in total body iron stores. Humans, like most animals, have no means to excrete excess iron, with the exception of menstruation which, for the average woman, results in a loss of 3.2 mg of iron.
Liver function tests, also referred to as a hepatic panel, are groups of blood tests that provide information about the state of a patient's liver. These tests include prothrombin time (PT/INR), activated partial thromboplastin time (aPTT), albumin, bilirubin, and others. The liver transaminases aspartate transaminase and alanine transaminase are useful biomarkers of liver injury in a patient with some degree of intact liver function.

Ferritin is a universal intracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. It is the primary intracellular iron-storage protein in both prokaryotes and eukaryotes, keeping iron in a soluble and non-toxic form. In humans, it acts as a buffer against iron deficiency and iron overload.
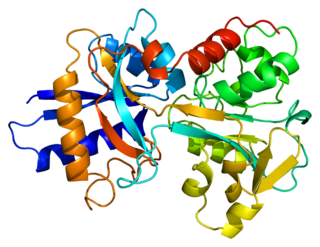
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded by the TF gene and produced as a 76 kDa glycoprotein.

Iron overload or haemochromatosis indicates increased total accumulation of iron in the body from any cause and resulting organ damage. The most important causes are hereditary haemochromatosis, a genetic disorder, and transfusional iron overload, which can result from repeated blood transfusions.
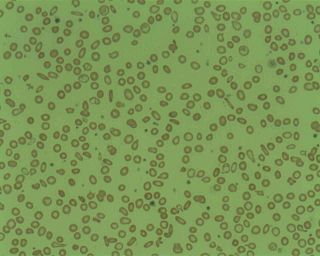
Microcytic anaemia is any of several types of anemia characterized by smaller than normal red blood cells. The normal mean corpuscular volume is approximately 80–100 fL. When the MCV is <80 fL, the red cells are described as microcytic and when >100 fL, macrocytic. The MCV is the average red blood cell size.
Serum iron is a medical laboratory test that measures the amount of circulating iron that is bound to transferrin and freely circulate in the blood. Clinicians order this laboratory test when they are concerned about iron deficiency, which can cause anemia and other problems. 65% of the iron in the body is bound up in hemoglobin molecules in red blood cells. About 4% is bound up in myoglobin molecules. Around 30% of the iron in the body is stored as ferritin or hemosiderin in the spleen, the bone marrow and the liver. Small amounts of iron can be found in other molecules in cells throughout the body. None of this iron is directly accessible by testing the serum.}
Transferrin saturation (TS), measured as a percentage, is a medical laboratory value. It is the value of serum iron divided by the total iron-binding capacity of the available transferrin, the main protein that binds iron in the blood, this value tells a clinician how much serum iron is bound. For instance, a value of 15% means that 15% of iron-binding sites of transferrin are being occupied by iron. The three results are usually reported together. A low transferrin saturation is a common indicator of iron deficiency anemia whereas a high transferrin saturation may indicate iron overload or hemochromatosis. Transferrin saturation is also called transferrin saturation index (TSI) or transferrin saturation percentage (TS%)

Human iron metabolism is the set of chemical reactions that maintain human homeostasis of iron at the systemic and cellular level. Iron is both necessary to the body and potentially toxic. Controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism, because iron is essential for red blood cells, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron-deficiency anemia.

African iron overload is an iron overload disorder first observed among people of African descent in Southern Africa and Central Africa. It is now recognized to actually be two disorders with different causes, possibly compounding each other:

Atransferrinemia is an autosomal recessive metabolic disorder in which there is an absence of transferrin, a plasma protein that transports iron through the blood. Atransferrinemia is characterized by anemia and hemosiderosis in the heart and liver. The iron damage to the heart can lead to heart failure. The anemia is typically microcytic and hypochromic. Atransferrinemia was first described in 1961 and is extremely rare, with only ten documented cases worldwide.
Juvenile hemochromatosis, also known as hemochromatosis type 2, is a rare form of hereditary hemochromatosis, which emerges in young individuals, typically between 15 and 30 years of age, but occasionally later. It is characterized by an inability to control how much iron is absorbed by the body, in turn leading to iron overload, where excess iron accumulates in many areas of the body and causes damage to the places it accumulates.

Hemosiderosis is a form of iron overload disorder resulting in the accumulation of hemosiderin.
Haemochromatosis type 3 is a type of iron overload disorder associated with deficiencies in transferrin receptor 2. It exhibits an autosomal recessive inheritance pattern. The first confirmed case was diagnosed in 1865 by French doctor Trousseau. Later in 1889, the German doctor von Recklinghausen indicated that the liver contains iron, and due to bleeding being considered to be the cause, he called the pigment "Haemochromatosis." In 1935, English doctor Sheldon's groundbreaking book titled, Haemochromatosis, reviewed 311 patient case reports and presented the idea that haemochromatosis was a congenital metabolic disorder. Hereditary haemochromatosis is a congenital disorder which affects the regulation of iron metabolism thus causing increased gut absorption of iron and a gradual build-up of pathologic iron deposits in the liver and other internal organs, joint capsules and the skin. The iron overload could potentially cause serious disease from the age of 40–50 years. In the final stages of the disease, the major symptoms include liver cirrhosis, diabetes and bronze-colored skin. There are four types of hereditary hemochromatosis which are classified depending on the age of onset and other factors such as genetic cause and mode of inheritance.
Soluble transferrin receptor conventionally refers to the cleaved extracellular portion of transferrin receptor 1 that is released into serum. This receptor is a protein dimer of two identical subunits, linked together by two pairs of disulfide bonds. Its molecular mass 190,000 Dalton.
Iron tests are groups of clinical chemistry laboratory blood tests that are used to evaluate body iron stores or the iron level in blood serum.
Latent iron deficiency (LID), also called iron-deficient erythropoiesis, is a medical condition in which there is evidence of iron deficiency without anemia. It is important to assess this condition because individuals with latent iron deficiency may develop iron-deficiency anemia. Additionally, there is some evidence of a decrease in vitality and an increase in fatigue among individuals with LID.
Hemochromatosis type 4 is a hereditary iron overload disorder that affects ferroportin, an iron transport protein needed to export iron from cells into circulation. Although the disease is rare, it is found throughout the world and affects people from various ethnic groups. While the majority of individuals with type 4 hemochromatosis have a relatively mild form of the disease, some affected individuals have a more severe form. As the disease progresses, iron may accumulate in the tissues of affected individuals over time, potentially resulting in organ damage.

The HFE H63D is a single-nucleotide polymorphism in the HFE gene, which results in the substitution of a histidine for an aspartic acid at amino acid position 63 of the HFE protein (p.His63Asp). HFE participates in the regulation of iron absorption.
Iron preparation is the formulation for iron supplements indicated in prophylaxis and treatment of iron-deficiency anemia. Examples of iron preparation include ferrous sulfate, ferrous gluconate, and ferrous fumarate. It can be administered orally, and by intravenous injection, or intramuscular injection.










