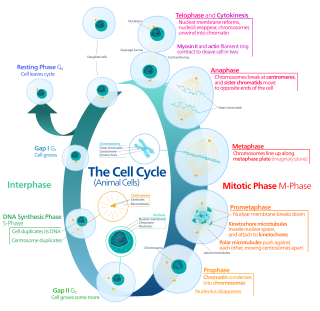
Mitosis is a part of the cell cycle in which replicated chromosomes are separated into two new nuclei. Cell division by mitosis is an equational division which gives rise to genetically identical cells in which the total number of chromosomes is maintained. Mitosis is preceded by the S phase of interphase and is followed by telophase and cytokinesis, which divide the cytoplasm, organelles, and cell membrane of one cell into two new cells containing roughly equal shares of these cellular components. The different stages of mitosis altogether define the mitotic phase of a cell cycle—the division of the mother cell into two daughter cells genetically identical to each other.

Cell division is the process by which a parent cell divides into two daughter cells. Cell division usually occurs as part of a larger cell cycle in which the cell grows and replicates its chromosome(s) before dividing. In eukaryotes, there are two distinct types of cell division: a vegetative division (mitosis), producing daughter cells genetically identical to the parent cell, and a cell division that produces haploid gametes for sexual reproduction (meiosis), reducing the number of chromosomes from two of each type in the diploid parent cell to one of each type in the daughter cells. Mitosis is a part of the cell cycle, in which, replicated chromosomes are separated into two new nuclei. Cell division gives rise to genetically identical cells in which the total number of chromosomes is maintained. In general, mitosis is preceded by the S stage of interphase and is followed by telophase and cytokinesis; which divides the cytoplasm, organelles, and cell membrane of one cell into two new cells containing roughly equal shares of these cellular components. The different stages of mitosis all together define the M phase of an animal cell cycle—the division of the mother cell into two genetically identical daughter cells. To ensure proper progression through the cell cycle, DNA damage is detected and repaired at various checkpoints throughout the cycle. These checkpoints can halt progression through the cell cycle by inhibiting certain cyclin-CDK complexes. Meiosis undergoes two divisions resulting in four haploid daughter cells. Homologous chromosomes are separated in the first division of meiosis, such that each daughter cell has one copy of each chromosome. These chromosomes have already been replicated and have two sister chromatids which are then separated during the second division of meiosis. Both of these cell division cycles are used in the process of sexual reproduction at some point in their life cycle. Both are believed to be present in the last eukaryotic common ancestor.

In cell biology, the centrosome is an organelle that serves as the main microtubule organizing center (MTOC) of the animal cell, as well as a regulator of cell-cycle progression. The centrosome provides structure for the cell. The centrosome is thought to have evolved only in the metazoan lineage of eukaryotic cells. Fungi and plants lack centrosomes and therefore use other structures to organize their microtubules. Although the centrosome has a key role in efficient mitosis in animal cells, it is not essential in certain fly and flatworm species.

In cell biology, the spindle apparatus is the cytoskeletal structure of eukaryotic cells that forms during cell division to separate sister chromatids between daughter cells. It is referred to as the mitotic spindle during mitosis, a process that produces genetically identical daughter cells, or the meiotic spindle during meiosis, a process that produces gametes with half the number of chromosomes of the parent cell.

Telophase is the final stage in both meiosis and mitosis in a eukaryotic cell. During telophase, the effects of prophase and prometaphase are reversed. As chromosomes reach the cell poles, a nuclear envelope is re-assembled around each set of chromatids, the nucleoli reappear, and chromosomes begin to decondense back into the expanded chromatin that is present during interphase. The mitotic spindle is disassembled and remaining spindle microtubules are depolymerized. Telophase accounts for approximately 2% of the cell cycle's duration.

The spindle checkpoint, also known as the metaphase-to-anaphase transition, the spindle assembly checkpoint (SAC), the metaphase checkpoint, or the mitotic checkpoint, is a cell cycle checkpoint during metaphase of mitosis or meiosis that prevents the separation of the duplicated chromosomes (anaphase) until each chromosome is properly attached to the spindle. To achieve proper segregation, the two kinetochores on the sister chromatids must be attached to opposite spindle poles. Only this pattern of attachment will ensure that each daughter cell receives one copy of the chromosome. The defining biochemical feature of this checkpoint is the stimulation of the anaphase-promoting complex by M-phase cyclin-CDK complexes, which in turn causes the proteolytic destruction of cyclins and proteins that hold the sister chromatids together.

A kinetochore is a disc-shaped protein structure associated with duplicated chromatids in eukaryotic cells where the spindle fibers attach during cell division to pull sister chromatids apart. The kinetochore assembles on the centromere and links the chromosome to microtubule polymers from the mitotic spindle during mitosis and meiosis. The term kinetochore was first used in a footnote in a 1934 Cytology book by Lester W. Sharp and commonly accepted in 1936. Sharp's footnote reads: "The convenient term kinetochore has been suggested to the author by J. A. Moore", likely referring to John Alexander Moore who had joined Columbia University as a freshman in 1932.

Aurora kinase B is a protein that functions in the attachment of the mitotic spindle to the centromere.

Aurora kinase inhibitors are a putative drug class for treating cancer. The Aurora kinase enzymes could be potential targets for novel small-molecule enzyme inhibitors.
Polo-like kinases (Plks) are regulatory serine/threonine kinases of the cell cycle involved in mitotic entry, mitotic exit, spindle formation, cytokinesis, and meiosis. Only one Plk is found in the genomes of the fly Drosophila melanogaster (Polo), budding yeast (Cdc5) and fission yeast (Plo1). Vertebrates and other animals, however, have many Plk family members including Plk1, Plk2/Snk, Plk3/Prk/FnK, Plk4/Sak and Plk5. Of the vertebrate Plk family members, the mammalian Plk1 has been most extensively studied. During mitosis and cytokinesis, Plks associate with several structures including the centrosome, kinetochores, and the central spindle.

Serine/threonine-protein kinase PLK1, also known as polo-like kinase 1 (PLK-1) or serine/threonine-protein kinase 13 (STPK13), is an enzyme that in humans is encoded by the PLK1 gene.

Serine/threonine-protein kinase Nek2 is an enzyme that in humans is encoded by the NEK2 gene.
Kinesin-like protein KIF23 is a protein that in humans is encoded by the KIF23 gene.

Inner centromere protein is a protein that in humans is encoded by the INCENP gene. It is a regulatory protein in the chromosome passenger complex (CPC). It is involved in regulation of the catalytic proteins Aurora B and Aurora C. It acts in association with two other proteins - Survivin and Borealin. These proteins form a tight three-helical bundle. The N-terminal domain of INCENP is the domain involved in formation of this three-helical bundle while its C-terminal domain is responsible for the interaction with Aurora B.

Targeting protein for Xklp2 is a protein that in humans is encoded by the TPX2 gene. It is one of the many spindle assembly factors that play a key role in inducing microtubule assembly and growth during M phase.

Aurora kinase C, also Serine/threonine-protein kinase 13 is an enzyme that in humans is encoded by the AURKC gene.

Cytoskeleton-associated protein 5 is a microtubule-associated protein that in humans is encoded by the CKAP5 gene. It is the homolog of the Xenopus protein XMAP215 and is also known as ch-Tog.
Cdc14 and Cdc14 are a gene and its protein product respectively. Cdc14 is found in most of the eukaryotes. Cdc14 was defined by Hartwell in his famous screen for loci that control the cell cycle of Saccharomyces cerevisiae. Cdc14 was later shown to encode a protein phosphatase. Cdc14 is dual-specificity, which means it has serine/threonine and tyrosine-directed activity. A preference for serines next to proline is reported. Many early studies, especially in the budding yeast Saccharomyces cerevisiae, demonstrated that the protein plays a key role in regulating late mitotic processes. However, more recent work in a range of systems suggests that its cellular function is more complex.

Centrosomes are the major microtubule organizing centers (MTOC) in mammalian cells. Failure of centrosome regulation can cause mistakes in chromosome segregation and is associated with aneuploidy. A centrosome is composed of two orthogonal cylindrical protein assemblies, called centrioles, which are surrounded by a protein dense amorphous cloud of pericentriolar material (PCM). The PCM is essential for nucleation and organization of microtubules. The centrosome cycle is important to ensure that daughter cells receive a centrosome after cell division. As the cell cycle progresses, the centrosome undergoes a series of morphological and functional changes. Initiation of the centrosome cycle occurs early in the cell cycle in order to have two centrosomes by the time mitosis occurs.

Kinesin-like protein KIF11 is a molecular motor protein that is essential in mitosis. In humans it is coded for by the gene KIF11. Kinesin-like protein KIF11 is a member of the kinesin superfamily, which are nanomotors that move along microtubule tracks in the cell. Named from studies in the early days of discovery, it is also known as Kinesin-5, or as BimC, Eg5 or N-2, based on the founding members of this kinesin family.