Related Research Articles

Cardiac glycosides are a class of organic compounds that increase the output force of the heart and decrease its rate of contractions by inhibiting the cellular sodium-potassium ATPase pump. Their beneficial medical uses are as treatments for congestive heart failure and cardiac arrhythmias; however, their relative toxicity prevents them from being widely used. Most commonly found as secondary metabolites in several plants such as foxglove plants, these compounds nevertheless have a diverse range of biochemical effects regarding cardiac cell function and have also been suggested for use in cancer treatment.

An action potential occurs when the membrane potential of a specific cell rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and in some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells.

The contraction of cardiac muscle in all animals is initiated by electrical impulses known as action potentials that in the heart are known as cardiac action potentials. The rate at which these impulses fire controls the rate of cardiac contraction, that is, the heart rate. The cells that create these rhythmic impulses, setting the pace for blood pumping, are called pacemaker cells, and they directly control the heart rate. They make up the cardiac pacemaker, that is, the natural pacemaker of the heart. In most humans, the highest concentration of pacemaker cells is in the sinoatrial (SA) node, the natural and primary pacemaker, and the resultant rhythm is a sinus rhythm.
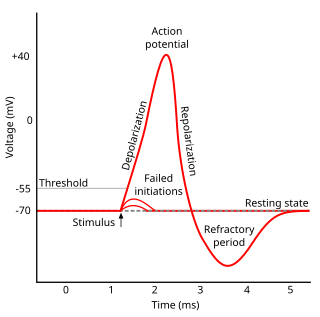
Refractoriness is the fundamental property of any object of autowave nature not responding to stimuli, if the object stays in the specific refractory state. In common sense, refractory period is the characteristic recovery time, a period that is associated with the motion of the image point on the left branch of the isocline .
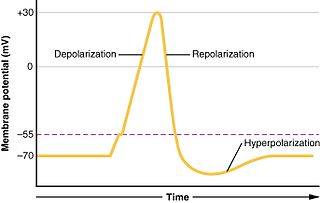
In biology, depolarization or hypopolarization is a change within a cell, during which the cell undergoes a shift in electric charge distribution, resulting in less negative charge inside the cell compared to the outside. Depolarization is essential to the function of many cells, communication between cells, and the overall physiology of an organism.

Membrane potential is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charges to move from the internal to exterior cellular environments and vice versa, as long as there is no acquisition of kinetic energy or the production of radiation. The concentration gradients of the charges directly determine this energy requirement. For the exterior of the cell, typical values of membrane potential, normally given in units of milli volts and denoted as mV, range from –80 mV to –40 mV.

In electrophysiology, the threshold potential is the critical level to which a membrane potential must be depolarized to initiate an action potential. In neuroscience, threshold potentials are necessary to regulate and propagate signaling in both the central nervous system (CNS) and the peripheral nervous system (PNS).
An inotrope or inotropic is a drug or any substance that alters the force or energy of muscular contractions. Negatively inotropic agents weaken the force of muscular contractions. Positively inotropic agents increase the strength of muscular contraction.
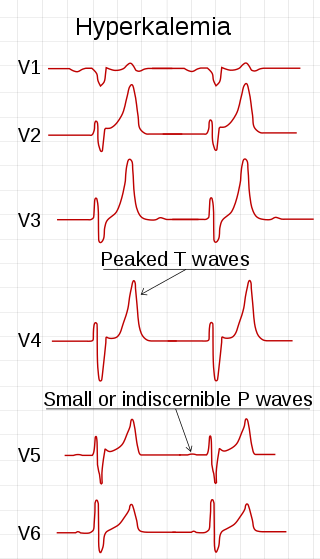
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5 mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death.

The cardiac action potential is a brief change in voltage across the cell membrane of heart cells. This is caused by the movement of charged atoms between the inside and outside of the cell, through proteins called ion channels. The cardiac action potential differs from action potentials found in other types of electrically excitable cells, such as nerves. Action potentials also vary within the heart; this is due to the presence of different ion channels in different cells.
Hyperkalemic periodic paralysis is an inherited autosomal dominant disorder that affects sodium channels in muscle cells and the ability to regulate potassium levels in the blood. It is characterized by muscle hyperexcitability or weakness which, exacerbated by potassium, heat or cold, can lead to uncontrolled shaking followed by paralysis. Onset usually occurs in early childhood, but it still occurs with adults.
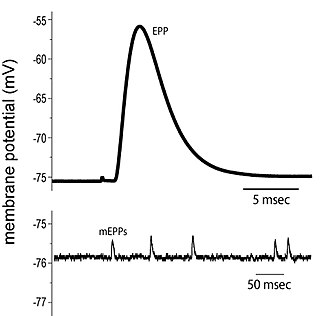
End plate potentials (EPPs) are the voltages which cause depolarization of skeletal muscle fibers caused by neurotransmitters binding to the postsynaptic membrane in the neuromuscular junction. They are called "end plates" because the postsynaptic terminals of muscle fibers have a large, saucer-like appearance. When an action potential reaches the axon terminal of a motor neuron, vesicles carrying neurotransmitters are exocytosed and the contents are released into the neuromuscular junction. These neurotransmitters bind to receptors on the postsynaptic membrane and lead to its depolarization. In the absence of an action potential, acetylcholine vesicles spontaneously leak into the neuromuscular junction and cause very small depolarizations in the postsynaptic membrane. This small response (~0.4mV) is called a miniature end plate potential (MEPP) and is generated by one acetylcholine-containing vesicle. It represents the smallest possible depolarization which can be induced in a muscle.
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels.
Myocardial contractility represents the innate ability of the heart muscle (cardiac muscle or myocardium) to contract. The ability to produce changes in force during contraction result from incremental degrees of binding between different types of tissue, that is, between filaments of myosin (thick) and actin (thin) tissue. The degree of binding depends upon the concentration of calcium ions in the cell. Within an in vivo intact heart, the action/response of the sympathetic nervous system is driven by precisely timed releases of a catecholamine, which is a process that determines the concentration of calcium ions in the cytosol of cardiac muscle cells. The factors causing an increase in contractility work by causing an increase in intracellular calcium ions (Ca++) during contraction.
Sodium channel blockers are drugs which impair the conduction of sodium ions (Na+) through sodium channels.
Potassium binders are medications that bind potassium ions in the gastrointestinal tract, thereby preventing its intestinal absorption. This category formerly consisted solely of polystyrene sulfonate, a polyanionic resin attached to a cation, administered either orally or by retention enema to patients who are at risk of developing hyperkalaemia. Newer drugs include: another polyanionic polymer, patiromer, which exchanges calcium for potassium; and Sodium zirconium cyclosilicate crystals, which exchange sodium for potassium
Cellular neuroscience is a branch of neuroscience concerned with the study of neurons at a cellular level. This includes morphology and physiological properties of single neurons. Several techniques such as intracellular recording, patch-clamp, and voltage-clamp technique, pharmacology, confocal imaging, molecular biology, two photon laser scanning microscopy and Ca2+ imaging have been used to study activity at the cellular level. Cellular neuroscience examines the various types of neurons, the functions of different neurons, the influence of neurons upon each other, and how neurons work together.

Nonsynaptic plasticity is a form of neuroplasticity that involves modification of ion channel function in the axon, dendrites, and cell body that results in specific changes in the integration of excitatory postsynaptic potentials and inhibitory postsynaptic potentials. Nonsynaptic plasticity is a modification of the intrinsic excitability of the neuron. It interacts with synaptic plasticity, but it is considered a separate entity from synaptic plasticity. Intrinsic modification of the electrical properties of neurons plays a role in many aspects of plasticity from homeostatic plasticity to learning and memory itself. Nonsynaptic plasticity affects synaptic integration, subthreshold propagation, spike generation, and other fundamental mechanisms of neurons at the cellular level. These individual neuronal alterations can result in changes in higher brain function, especially learning and memory. However, as an emerging field in neuroscience, much of the knowledge about nonsynaptic plasticity is uncertain and still requires further investigation to better define its role in brain function and behavior.
Cardiac physiology or heart function is the study of healthy, unimpaired function of the heart: involving blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output and how these interact and depend on one another.
Goniopora toxin (GPT) is a polypeptide toxin from the marine Goniopora species coral. Two toxins from this source have been identified, one acting on sodium channels and one acting on calcium channels.
References
- ↑ Miriam Webster's Medical Dictionary and Online Medical Dictionary
- ↑ "The Kanji Foundry Press - b". Archived from the original on 2008-03-12. Retrieved 2008-05-14.
- ↑ "Bathmotropy".
- ↑ Engelmann, Th. W. (January 1897). "Ueber den myogenen Ursprung der Herzthätigkeit und über automatische Erregbarkeit als normale Eigenschaft peripherischer Nervenfasern". Pflügers Archiv (in German). 65 (11–12): 535–578. doi:10.1007/BF01795562. ISSN 1432-2013. S2CID 31891993.
- ↑ Katz AM; Smith VE (1982). "Inotropic and lusitropic abnormalities in the genesis of heart failure". Eur Heart J. 3 (Suppl D): 11–18. doi:10.1093/eurheartj/4.suppl_a.7. PMID 6220901.
- ↑ The American Journal of the Medical Sciences. J.B. Lippincott, Company. 1908. pp. 46–.
- ↑ Scientific American Medical; Dale and Federman Vol 1; 2003 Edition p. 1907 chapter 160; Disorders of Acid-Base and Potassium Balance
- 1 2 Armstrong, C.M.; Cota, Gabriel. (1999). "Calcium block of Na+ channels and its effect on closing rate". Proceedings of the National Academy of Sciences of the United States of America . 96 (7): 4154–4157. Bibcode:1999PNAS...96.4154A. doi: 10.1073/pnas.96.7.4154 . PMC 22436 . PMID 10097179.
- ↑ Kahloon, Mansha U.; Aslam, Ahmad K.; Aslam, Ahmed F.; Wilbur, Sabrina L.; Vasavada, Balendu C.; Khan, Ijaz A. (November 2005). "Hyperkalemia induced failure of atrial and ventricular pacemaker capture". International Journal of Cardiology. 105 (2): 224–226. doi:10.1016/j.ijcard.2004.11.028. PMID 16243117.
- ↑ Veldkamp, M (1 June 2001). "Norepinephrine induces action potential prolongation and early afterdepolarizations in ventricular myocytes isolated from human end-stage failing hearts". European Heart Journal. 22 (11): 955–963. doi: 10.1053/euhj.2000.2499 . PMID 11428819.
- ↑ Ebner, F; Reiter, M (June 1979). "The alteration by propranolol of the inotropic and bathmotropic effects of dihydro-ouabain on guinea-pig papillary muscle". Naunyn-Schmiedeberg's Archives of Pharmacology. 307 (2): 99–104. doi:10.1007/BF00498450. PMID 481617. S2CID 26706163.
- ↑ Hypokalemia