Gout is a form of inflammatory arthritis characterized by recurrent attacks of pain in a red, tender, hot, and swollen joint, caused by the deposition of needle-like crystals of uric acid known as monosodium urate crystals. Pain typically comes on rapidly, reaching maximal intensity in less than 12 hours. The joint at the base of the big toe is affected (Podagra) in about half of cases. It may also result in tophi, kidney stones, or kidney damage.
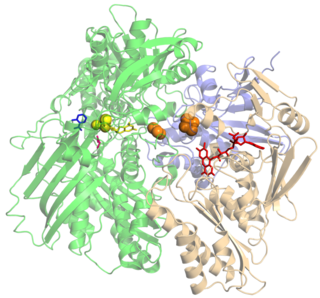
Xanthine oxidase is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans.

Allopurinol is a medication used to decrease high blood uric acid levels. It is specifically used to prevent gout, prevent specific types of kidney stones and for the high uric acid levels that can occur with chemotherapy. It is taken orally or intravenously.
Hyperuricaemia or hyperuricemia is an abnormally high level of uric acid in the blood. In the pH conditions of body fluid, uric acid exists largely as urate, the ion form. Serum uric acid concentrations greater than 6 mg/dL for females, 7 mg/dL for men, and 5.5 mg/dL for youth are defined as hyperuricemia. The amount of urate in the body depends on the balance between the amount of purines eaten in food, the amount of urate synthesised within the body, and the amount of urate that is excreted in urine or through the gastrointestinal tract. Hyperuricemia may be the result of increased production of uric acid, decreased excretion of uric acid, or both increased production and reduced excretion.

Naproxen, sold under the brand name Aleve among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain, menstrual cramps, and inflammatory diseases such as rheumatoid arthritis, gout and fever. It is taken orally. It is available in immediate and delayed release formulations. Onset of effects is within an hour and lasts for up to twelve hours.

Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers.

Doxepin is a medication belonging to the tricyclic antidepressant (TCA) class of drugs used to treat major depressive disorder, anxiety disorders, chronic hives, and insomnia. For hives it is a less preferred alternative to antihistamines. It has a mild to moderate benefit for sleeping problems. It is used as a cream for itchiness due to atopic dermatitis or lichen simplex chronicus.
Uricosuric medications (drugs) are substances that increase the excretion of uric acid in the urine, thus reducing the concentration of uric acid in blood plasma. In general, this effect is achieved by action on the proximal tubule of the kidney. Drugs that reduce blood uric acid are not all uricosurics; blood uric acid can be reduced by other mechanisms.
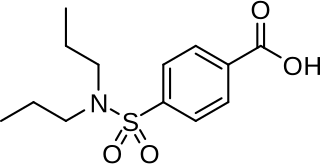
Probenecid, also sold under the brand name Probalan, is a medication that increases uric acid excretion in the urine. It is primarily used in treating gout and hyperuricemia.

Cytochrome P450 family 2 subfamily C member 9 is an enzyme protein. The enzyme is involved in the metabolism, by oxidation, of both xenobiotics, including drugs, and endogenous compounds, including fatty acids. In humans, the protein is encoded by the CYP2C9 gene. The gene is highly polymorphic, which affects the efficiency of the metabolism by the enzyme.

Cytochrome P450 2C19 is an enzyme protein. It is a member of the CYP2C subfamily of the cytochrome P450 mixed-function oxidase system. This subfamily includes enzymes that catalyze metabolism of xenobiotics, including some proton pump inhibitors and antiepileptic drugs. In humans, it is the CYP2C19 gene that encodes the CYP2C19 protein. CYP2C19 is a liver enzyme that acts on at least 10% of drugs in current clinical use, most notably the antiplatelet treatment clopidogrel (Plavix), drugs that treat pain associated with ulcers, such as omeprazole, antiseizure drugs such as mephenytoin, the antimalarial proguanil, and the anxiolytic diazepam.

Febuxostat, sold under the brand names Uloric among others, is a medication used long-term to treat gout due to high uric acid levels. It is generally recommended only for people who cannot take allopurinol. When initially started, medications such as NSAIDs are often recommended to prevent gout flares. It is taken by mouth.

Hyperuricosuria is a medical term referring to the presence of excessive amounts of uric acid in the urine. For men this is at a rate greater than 800 mg/day, and for women, 750 mg/day. Notable direct causes of hyperuricosuria are dissolution of uric acid crystals in the kidneys or urinary bladder, and hyperuricemia. Notable indirect causes include uricosuric drugs, rapid breakdown of bodily tissues containing large quantities of DNA and RNA, and a diet high in purine.
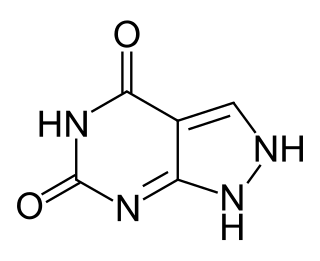
Oxipurinol is an inhibitor of xanthine oxidase. It is an active metabolite of allopurinol and it is cleared renally. In cases of renal disease, this metabolite will accumulate to toxic levels. By inhibiting xanthine oxidase, it reduces uric acid production. High serum uric acid levels may result in gout, kidney stones, and other medical conditions.
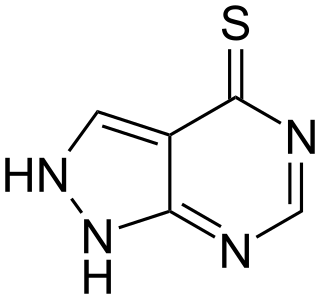
Tisopurine is a drug used in the treatment of gout in some countries. It reduces uric acid production through inhibiting an early stage in its production.
A xanthine oxidase inhibitor is any substance that inhibits the activity of xanthine oxidase, an enzyme involved in purine metabolism. In humans, inhibition of xanthine oxidase reduces the production of uric acid, and several medications that inhibit xanthine oxidase are indicated for treatment of hyperuricemia and related medical conditions including gout. Xanthine oxidase inhibitors are being investigated for management of reperfusion injury.
Tsai-Fan Yu was a Chinese-American physician, researcher, and the first woman to be appointed as a full professor at Mount Sinai School of Medicine. She helped to develop an explanation for the cause of gout and experimented with early drugs to treat the disease which are still in use today.

Lesinurad is a urate transporter inhibitor for treating high blood uric acid levels associated with gout. It is recommended only as an adjuvant with either allopurinol or febuxostat when these medications are not sufficient.

Nordoxepin, also known as N-desmethyldoxepin, is an organic compound. A colorless solid, it attracted attention as the major active metabolite of the tricyclic antidepressant (TCA) doxepin (Sinequan). It has been found to play a significant role in the antidepressant effects of doxepin.

Gout suppressants are agents which control and prevent gout attacks after the first episode. They can be generally classified into two groups by their purpose: drugs used for induction therapy and that for maintenance therapy.