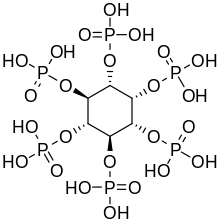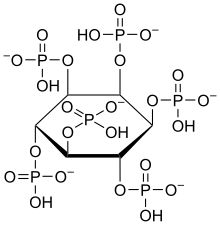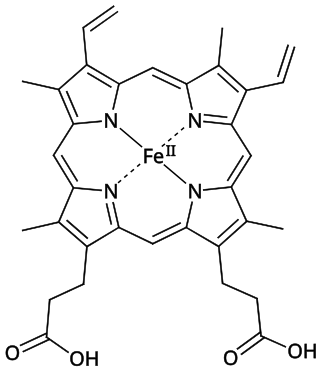
Iron deficiency, or sideropenia, is the state in which a body lacks enough iron to supply its needs. Iron is present in all cells in the human body and has several vital functions, such as carrying oxygen to the tissues from the lungs as a key component of the hemoglobin protein, acting as a transport medium for electrons within the cells in the form of cytochromes, and facilitating oxygen enzyme reactions in various tissues. Too little iron can interfere with these vital functions and lead to morbidity and death.

Sprouting is the natural process by which seeds or spores germinate and put out shoots, and already established plants produce new leaves or buds, or other structures experience further growth.

Bran, also known as miller's bran, is the hard layers of cereal grain surrounding the endosperm. It consists of the combined aleurone and pericarp. Corn (maize) bran also includes the pedicel. Along with the germ, it is an integral part of whole grains, and is often produced as a byproduct of milling in the production of refined grains.

Macrotyloma uniflorum is a legume native to tropical southern Asia, known for its distinct taste and texture, widely used legume in many cuisines. It is also known for human consumption for its rich nutrients and reputed medicinal properties. It is commonly grown for horse feed, hence the name “horse gram”. Horse gram grown in parts of India, as well as Nepal, Malaysia, Sri Lanka, and is introduced to the West Indies. It is consumed whole, sprouted, or ground. It is consumed in many parts of India and is also known as a superfood. Horse gram is also allowed to be eaten on some Hindu fasting days. Medical uses of these legumes have been discussed and is described in the Ayurveda.

Inositol, primarily the isomer myo-inositol, is a carbocyclic sugar that is abundant in the brain and other mammalian tissues; it mediates cell signal transduction in response to a variety of hormones, neurotransmitters, and growth factors and participates in osmoregulation. Concerning regulation of osmosis, in most mammalian cells the intracellular concentrations of myo-inositol are 5 to 500 times greater than the extracellular concentrations.

Vegetarian nutrition is the set of health-related challenges and advantages of vegetarian diets.

Inositol phosphates are a group of mono- to hexaphosphorylated inositols. Each form of inositol phosphate is distinguished by the number and position of the phosphate group on the inositol ring.

A phytase is any type of phosphatase enzyme that catalyzes the hydrolysis of phytic acid – an indigestible, organic form of phosphorus that is found in many plant tissues, especially in grains and oil seeds – and releases a usable form of inorganic phosphorus. While phytases have been found to occur in animals, plants, fungi and bacteria, phytases have been most commonly detected and characterized from fungi.

Inositol oxygenase, also commonly referred to as myo-inositol oxygenase (MIOX), is a non-heme di-iron enzyme that oxidizes myo-inositol to glucuronic acid. The enzyme employs a unique four-electron transfer at its Fe(II)/Fe(III) coordination sites and the reaction proceeds through the direct binding of myo-inositol followed by attack of the iron center by diatomic oxygen. This enzyme is part of the only known pathway for the catabolism of inositol in humans and is expressed primarily in the kidneys. Recent medical research regarding MIOX has focused on understanding its role in metabolic and kidney diseases such as diabetes, obesity and acute kidney injury. Industrially-focused engineering efforts are centered on improving MIOX activity in order to produce glucaric acid in heterologous hosts.
Zinc deficiency is defined either as insufficient zinc to meet the needs of the body, or as a serum zinc level below the normal range. However, since a decrease in the serum concentration is only detectable after long-term or severe depletion, serum zinc is not a reliable biomarker for zinc status. Common symptoms include increased rates of diarrhea. Zinc deficiency affects the skin and gastrointestinal tract; brain and central nervous system, immune, skeletal, and reproductive systems.
The enzyme phosphatidylinositol diacylglycerol-lyase catalyzes the following reaction:
The enzyme 3-phytase (EC 3.1.3.8) catalyzes the reaction
The enzyme 4-phytase (EC 3.1.3.26) catalyzes the following reaction:
The enzyme 5-phytase (EC 3.1.3.72) catalyzes the reaction

Antinutrients are natural or synthetic compounds that interfere with the absorption of nutrients. Nutrition studies focus on antinutrients commonly found in food sources and beverages. Antinutrients may take the form of drugs, chemicals that naturally occur in food sources, proteins, or overconsumption of nutrients themselves. Antinutrients may act by binding to vitamins and minerals, preventing their uptake, or inhibiting enzymes.

Nutritional neuroscience is the scientific discipline that studies the effects various components of the diet such as minerals, vitamins, protein, carbohydrates, fats, dietary supplements, synthetic hormones, and food additives have on neurochemistry, neurobiology, behavior, and cognition.
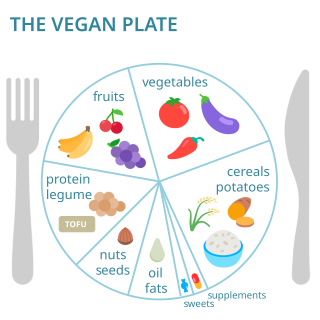
Vegan nutrition refers to the nutritional and human health aspects of vegan diets. A well-planned, balanced vegan diet is suitable to meet all recommendations for nutrients in every stage of human life. Vegan diets tend to be higher in dietary fiber, magnesium, folic acid, vitamin C, vitamin E, and phytochemicals; and lower in calories, saturated fat, iron, cholesterol, long-chain omega-3 fatty acids, vitamin D, calcium, zinc, and vitamin B12.
Inositol-hexakisphosphate kinase is an enzyme with systematic name ATP:1D-myo-inositol-hexakisphosphate 5-phosphotransferase. This enzyme catalyses the following chemical reaction
Soy formula is a substitute for human breast milk. It is a commercial product based on the proteins found in soybeans. Soy infant formula uses processed soybeans as its source of protein, and comes in powdered or liquid form. Usually lactose-free, soy infant formula contains a different sugar. Infants who are intolerant of cows' milk protein may also be intolerant of soy protein. It differs from human breast milk in a number of ways. Soy protein inhibits the absorption of iron. The soy-based formulas discussed by the World Health Organization reports that soy formula is fortified with iron to compensate for this effect. One naturally occurring plant-based compound found in soy-based infant formula is phytic acid. It is also a strong inhibitor of iron absorption, though it can be removed in processing. It is not known how many manufacturers of soy-based formula incorporate this practice. China and Vietnam have regulated soy-based infant formulas to include NaFeEDTA to fortify the formula and enhance the absorption of iron by the infant. When iron compounds are added to soy-based infant formula, the iron compound is encapsulated to prevent it from making the formula dark.

β-propeller phytases (BPPs) are a group of enzymes (i.e. protein superfamily) with a round beta-propeller structure. BPPs are phytases, which means that they are able to remove (hydrolyze) phosphate groups from phytic acid and its phytate salts. Hydrolysis happens stepwise and usually ends in myo-inositol triphosphate product which has three phosphate groups still bound to it. The actual substrate of BPPs is calcium phytate and in order to hydrolyze it, BPPs must have Ca2+ ions bound to themselves. BPPs are the most widely found phytase superfamily in the environment and they are thought to have a major role in phytate-phosphorus cycling in soil and water. As their alternative name alkaline phytase suggests, BPPs work best in basic (or neutral) environment. Their pH optima is 6–9, which is unique among the phytases.