GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases.

G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.
Ras, from "Rat sarcoma virus", is a family of related proteins that are expressed in all animal cell lineages and organs. All Ras protein family members belong to a class of protein called small GTPase, and are involved in transmitting signals within cells. Ras is the prototypical member of the Ras superfamily of proteins, which are all related in three-dimensional structure and regulate diverse cell behaviours.
Small GTPases, also known as small G-proteins, are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP). They are a type of G-protein found in the cytosol that are homologous to the alpha subunit of heterotrimeric G-proteins, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a guanosine triphosphate (GTP) to form guanosine diphosphate (GDP). The best-known members are the Ras GTPases and hence they are sometimes called Ras subfamily GTPases.

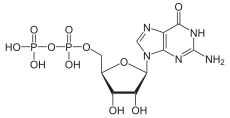
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon.
Transducin (Gt) is a protein naturally expressed in vertebrate retina rods and cones and it is very important in vertebrate phototransduction. It is a type of heterotrimeric G-protein with different α subunits in rod and cone photoreceptors.
A nucleoside triphosphate is a nucleoside containing a nitrogenous base bound to a 5-carbon sugar, with three phosphate groups bound to the sugar. They are the molecular precursors of both DNA and RNA, which are chains of nucleotides made through the processes of DNA replication and transcription. Nucleoside triphosphates also serve as a source of energy for cellular reactions and are involved in signalling pathways.
GTPase-activating proteins or GTPase-accelerating proteins (GAPs) are a family of regulatory proteins whose members can bind to activated G proteins and stimulate their GTPase activity, with the result of terminating the signaling event. GAPs are also known as RGS protein, or RGS proteins, and these proteins are crucial in controlling the activity of G proteins. Regulation of G proteins is important because these proteins are involved in a variety of important cellular processes. The large G proteins, for example, are involved in transduction of signaling from the G protein-coupled receptor for a variety of signaling processes like hormonal signaling, and small G proteins are involved in processes like cellular trafficking and cell cycling. GAP's role in this function is to turn the G protein's activity off. In this sense, GAPs function is opposite to that of guanine nucleotide exchange factors (GEFs), which serve to enhance G protein signaling.
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization.

Nucleotide exchange factors (NEFs) are proteins that stimulate the exchange (replacement) of nucleoside diphosphates for nucleoside triphosphates bound to other proteins.

ADP ribosylation factors (ARFs) are members of the ARF family of GTP-binding proteins of the Ras superfamily. ARF family proteins are ubiquitous in eukaryotic cells, and six highly conserved members of the family have been identified in mammalian cells. Although ARFs are soluble, they generally associate with membranes because of N-terminus myristoylation. They function as regulators of vesicular traffic and actin remodelling.

Ran also known as GTP-binding nuclear protein Ran is a protein that in humans is encoded by the RAN gene. Ran is a small 25 kDa protein that is involved in transport into and out of the cell nucleus during interphase and also involved in mitosis. It is a member of the Ras superfamily.
Nuclear transport refers to the mechanisms by which molecules move across the nuclear membrane of a cell. The entry and exit of large molecules from the cell nucleus is tightly controlled by the nuclear pore complexes (NPCs). Although small molecules can enter the nucleus without regulation, macromolecules such as RNA and proteins require association with transport factors known as nuclear transport receptors, like karyopherins called importins to enter the nucleus and exportins to exit.
In cell signalling, Son of Sevenless (SOS) refers to a set of genes encoding guanine nucleotide exchange factors that act on the Ras subfamily of small GTPases.

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.

GTP-binding protein regulators regulate G proteins in several different ways. Small GTPases act as molecular switches in signaling pathways, which act to regulate functions of other proteins. They are active or 'ON' when it is bound to GTP and inactive or 'OFF' when bound to GDP. Activation and deactivation of small GTPases can be regarded as occurring in a cycle, between the GTP-bound and GDP-bound form, regulated by other regulatory proteins.

GTPgammaS is a non-hydrolyzable or slowly hydrolyzable G-protein-activating analog of guanosine triphosphate (GTP). Many GTP binding proteins demonstrate activity when bound to GTP, and are inactivated via the hydrolysis of the phosphoanhydride bond that links the γ-phosphate to the remainder of the nucleotide, leaving a bound guanosine diphosphate (GDP) and releasing an inorganic phosphate. This usually occurs rapidly, and the GTP-binding protein can then only be activated by exchanging the GDP for a new GTP molecule. The substitution of sulfur for one of the oxygens of the γ-phosphate of GTP creates a nucleotide that either cannot be hydrolyzed or is only slowly hydrolyzed. This prevents the GTP-binding proteins from being inactivated, and allows the cellular processes that they carry out when active to be more easily studied.
G alpha subunits are one of the three types of subunit of guanine nucleotide binding proteins, which are membrane-associated, heterotrimeric G proteins.
EF-Ts is one of the prokaryotic elongation factors. It is found in human mitochondria as TSFM. It is similar to eukaryotic EF-1B.

EVI5L is a protein that in humans is encoded by the EVI5L gene. EVI5L is a member of the Ras superfamily of monomeric guanine nucleotide-binding (G) proteins, and functions as a GTPase-activating protein (GAP) with a broad specificity. Measurement of in vitro Rab-GAP activity has shown that EVI5L has significant Rab2A- and Rab10-GAP activity.