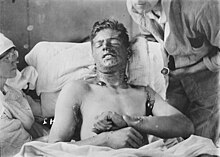
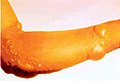
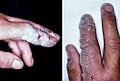
Mustard gas or sulfur mustard is any of several chemical compounds that contain the chemical structure S(CH2CH2Cl)2. In the wider sense, compounds with the substituent S(CH2CH2X)2 and N(CH2CH2X)3 are known as sulfur mustards and nitrogen mustards, respectively, where X = Cl or Br. Such compounds are potent alkylating agents, which can interfere with several biological processes. Also known as mustard agents, this family of compounds are infamous cytotoxins and blister agents with a long history of use as chemical weapons. The name mustard gas is technically incorrect: the substances, when dispersed, are often not gases but a fine mist of liquid droplets. Sulfur mustards are viscous liquids at room temperature and have an odor resembling mustard plants, garlic, or horseradish, hence the name. When pure, they are colorless, but when used in impure forms, such as in warfare, they are usually yellow-brown. Mustard gases form blisters on exposed skin and in the lungs, often resulting in prolonged illness ending in death. The typical mustard gas is the organosulfur compound bis(2-chloroethyl) sulfide.

Lewisite (L) (A-243) is an organoarsenic compound. It was once manufactured in the U.S., Japan, Germany and the Soviet Union for use as a chemical weapon, acting as a vesicant and lung irritant. Although the substance is colorless and odorless in its pure form, impure samples of lewisite are a yellow, brown, violet-black, green, or amber oily liquid with a distinctive odor that has been described as similar to geraniums.

A blister is a small pocket of body fluid within the upper layers of the skin, usually caused by forceful rubbing (friction), burning, freezing, chemical exposure or infection. Most blisters are filled with a clear fluid, either serum or plasma. However, blisters can be filled with blood or with pus.

Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers.

A blister agent, is a chemical compound that causes severe skin, eye and mucosal pain and irritation. They are named for their ability to cause severe chemical burns, resulting in painful water blisters on the bodies of those affected. Although the term is often used in connection with large-scale burns caused by chemical spills or chemical warfare agents, some naturally occurring substances such as cantharidin are also blister-producing agents (vesicants). Furanocoumarin, another naturally occurring substance, causes vesicant-like effects indirectly, for example, by increasing skin photosensitivity greatly. Vesicants have medical uses including wart removal but can be dangerous if even small amounts are ingested.

Chlormethine, also known as mechlorethamine, mustine, HN2, and embikhin (эмбихин), is a nitrogen mustard sold under the brand name Mustargen among others. It is the prototype of alkylating agents, a group of anticancer chemotherapeutic drugs. It works by binding to DNA, crosslinking two strands and preventing cell duplication. It binds to the N7 nitrogen on the DNA base guanine. As the chemical is a blister agent, its use is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance.

Bromine pentafluoride, BrF5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent.
Zinc peroxide (ZnO2) appears as a bright yellow powder at room temperature. It was historically used as a surgical antiseptic. More recently zinc peroxide has also been used as an oxidant in explosives and pyrotechnic mixtures. Its properties have been described as a transition between ionic and covalent peroxides. Zinc peroxide can be synthesized through the reaction of zinc chloride and hydrogen peroxide.

Phenyldichloroarsine, also known by its wartime name phenyl Dick and its NATO abbreviation PD, is an organic arsenical vesicant and vomiting agent developed by Germany and France for use as a chemical warfare agent during World War I. The agent is known by multiple synonyms and is technically classified as a vesicant, or blister agent.
Hazard statements form part of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). They are intended to form a set of standardized phrases about the hazards of chemical substances and mixtures that can be translated into different languages. As such, they serve the same purpose as the well-known R-phrases, which they are intended to replace.
Acute inhalation injury may result from frequent and widespread use of household cleaning agents and industrial gases. The airways and lungs receive continuous first-pass exposure to non-toxic and irritant or toxic gases via inhalation. Irritant gases are those that, on inhalation, dissolve in the water of the respiratory tract mucosa and provoke an inflammatory response, usually from the release of acidic or alkaline radicals. Smoke, chlorine, phosgene, sulfur dioxide, hydrogen chloride, hydrogen sulfide, nitrogen dioxide, ozone, and ammonia are common irritants.
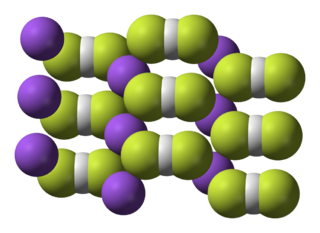
Sodium bifluoride is the inorganic compound with the formula Na[HF2]. It is a salt of sodium cation and bifluoride anion. It is a white, water-soluble solid that decomposes upon heating. Sodium bifluoride is non-flammable, hygroscopic, and has a pungent smell. Sodium bifluoride has a number of applications in industry.
Caustic ingestion occurs when someone accidentally or deliberately ingests a caustic or corrosive substance. Depending on the nature of the substance, the duration of exposure and other factors it can lead to varying degrees of damage to the oral mucosa, the esophagus, and the lining of the stomach.

A hydrofluoric acid burn is a chemical burn from hydrofluoric acid. Where it contacts the skin it results in significant pain, swelling, redness, and skin breakdown. If the fumes are breathed in swelling of the upper airway and bleeding may occur. Complications can include electrolyte, heart, lung, kidney, and neurological problems.

Diphoterine is a decontamination solution used in first aid for the emergency treatment of chemical spills to the eyes and body.
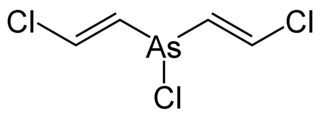
Lewisite 2(L-2) is an organoarsenic chemical weapon with the formula AsCl(CH=CHCl)2. It is similar to lewisite 1 and lewisite 3 and was first synthesized in 1904 by Julius Arthur Nieuwland. It is usually found as a mixture of 2-chlorovinylarsonous dichloride (lewisite 1) as well as bis(2-chloroethenyl) arsinous chloride (lewisite 2) and tris(2-chlorovinyl)arsine (lewisite 3). Pure lewisite 1 is an oily, colorless liquid, however, the impure mixture can appear amber to black with an odor distinct to geraniums.

Lewisite 3(L-3) is an organoarsenic chemical weapon like lewisite 1 and lewisite 2 first synthesized in 1904 by Julius Arthur Nieuwland. It is usually found as a mixture of 2-chlorovinylarsonous dichloride as well as bis(2-chloroethenyl) arsinous chloride and tris(2-chlorovinyl)arsine. Pure lewisite 1 is an oily, colorless liquid, however, the impure mixture can appear amber to black with an odor distinct to geraniums.
Chemical drain cleaners or openers are pure or mixtures of chemicals used to unclog drains that are blocked by hair, food, or other organic materials. They are often accompanied by other mechanical drain cleaners for the optimal effect. Chemical drain cleaners are available through hardware stores, although some may be intended for use by licensed plumbers. They may contain either strong acids or strong alkalis. These cleaners contain chemicals that dissolve at least some of the material causing the clog.

Bis(2-chloroethyl)sulfide is the organosulfur compound with the formula (ClCH2CH2)2S. It is a prominent member of a family of cytotoxic and blister agents known as mustard agents. Sometimes referred to as mustard gas, the term is technically incorrect: bis(2-chloroethyl)sulfide is a liquid at room temperature. In warfare it was dispersed in the form of a fine mist of liquid droplets.