Related Research Articles

The visual cortex of the brain is the area of the cerebral cortex that processes visual information. It is located in the occipital lobe. Sensory input originating from the eyes travels through the lateral geniculate nucleus in the thalamus and then reaches the visual cortex. The area of the visual cortex that receives the sensory input from the lateral geniculate nucleus is the primary visual cortex, also known as visual area 1 (V1), Brodmann area 17, or the striate cortex. The extrastriate areas consist of visual areas 2, 3, 4, and 5.
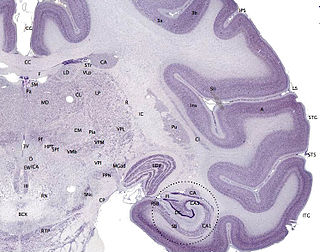
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex, with just 10% consisting of allocortex. It is separated into two cortices, by the longitudinal fissure that divides the cerebrum into the left and right cerebral hemispheres. The two hemispheres are joined beneath the cortex by the corpus callosum. The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is part of the brain responsible for cognition.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.

Retinotopy is the mapping of visual input from the retina to neurons, particularly those neurons within the visual stream. For clarity, 'retinotopy' can be replaced with 'retinal mapping', and 'retinotopic' with 'retinally mapped'.
Ocular dominance columns are stripes of neurons in the visual cortex of certain mammals that respond preferentially to input from one eye or the other. The columns span multiple cortical layers, and are laid out in a striped pattern across the surface of the striate cortex (V1). The stripes lie perpendicular to the orientation columns.
Fibroblast growth factors (FGF) are a family of cell signalling proteins produced by macrophages; they are involved in a wide variety of processes, most notably as crucial elements for normal development in animal cells. Any irregularities in their function lead to a range of developmental defects. These growth factors typically act as systemic or locally circulating molecules of extracellular origin that activate cell surface receptors. A defining property of FGFs is that they bind to heparin and to heparan sulfate. Thus, some are sequestered in the extracellular matrix of tissues that contains heparan sulfate proteoglycans and are released locally upon injury or tissue remodeling.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

Pasko Rakic is a Yugoslav-born American neuroscientist, who presently works in the Yale School of Medicine Department of Neuroscience in New Haven, Connecticut. His main research interest is in the development and evolution of the human brain. He was the founder and served as Chairman of the Department of Neurobiology at Yale, and was founder and Director of the Kavli Institute for Neuroscience. He is best known for elucidating the mechanisms involved in development and evolution of the cerebral cortex. In 2008, Rakic shared the inaugural Kavli Prize in Neuroscience. He is currently the Dorys McConell Duberg Professor of Neuroscience, leads an active research laboratory, and serves on Advisory Boards and Scientific Councils of a number of Institutions and Research Foundations.

The ganglionic eminence (GE) is a transitory structure in the development of the nervous system that guides cell and axon migration. It is present in the embryonic and fetal stages of neural development found between the thalamus and caudate nucleus.
Gyrification is the process of forming the characteristic folds of the cerebral cortex.
The Protomap is a primordial molecular map of the functional areas of the mammalian cerebral cortex during early embryonic development, at a stage when neural stem cells are still the dominant cell type. The protomap is a feature of the ventricular zone, which contains the principal cortical progenitor cells, known as radial glial cells. Through a process called 'cortical patterning', the protomap is patterned by a system of signaling centers in the embryo, which provide positional information and cell fate instructions. These early genetic instructions set in motion a development and maturation process that gives rise to the mature functional areas of the cortex, for example the visual, somatosensory, and motor areas. The term protomap was coined by Pasko Rakic. The protomap hypothesis was opposed by the protocortex hypothesis, which proposes that cortical proto-areas initially have the same potential, and that regionalization in large part is controlled by external influences, such as axonal inputs from the thalamus to the cortex. However, a series of papers in the year 2000 and in 2001 provided strong evidence against the protocortex hypothesis, and the protomap hypothesis has been well accepted since then. The protomap hypothesis, together with the related radial unit hypothesis, forms our core understanding of the embryonic development of the cerebral cortex. Once the basic structure is present and cortical neurons have migrated to their final destinations, many other processes contribute to the maturation of functional cortical circuits.
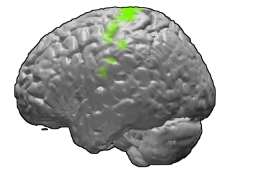
The primary motor cortex is a brain region that in humans is located in the dorsal portion of the frontal lobe. It is the primary region of the motor system and works in association with other motor areas including premotor cortex, the supplementary motor area, posterior parietal cortex, and several subcortical brain regions, to plan and execute voluntary movements. Primary motor cortex is defined anatomically as the region of cortex that contains large neurons known as Betz cells, which, along with other cortical neurons, send long axons down the spinal cord to synapse onto the interneuron circuitry of the spinal cord and also directly onto the alpha motor neurons in the spinal cord which connect to the muscles.

Eomesodermin also known as T-box brain protein 2 (Tbr2) is a protein that in humans is encoded by the EOMES gene.
Corticogenesis is the process during which the cerebral cortex of the brain is formed as part of the development of the nervous system of mammals including its development in humans. The cortex is the outer layer of the brain and is composed of up to six layers. Neurons formed in the ventricular zone migrate to their final locations in one of the six layers of the cortex. The process occurs from embryonic day 10 to 17 in mice and between gestational weeks seven to 18 in humans.
Cajal–Retzius cells are a heterogeneous population of morphologically and molecularly distinct reelin-producing cell types in the marginal zone/layer I of the developmental cerebral cortex and in the immature hippocampus of different species and at different times during embryogenesis and postnatal life.

Susan McConnell is a neurobiologist who studies the development of neural circuits in the mammalian cerebral cortex. She is a professor in the Department of Biology at Stanford University, where she is the Susan B. Ford Professor of Humanities and Sciences, a Bass University Fellow, and a Howard Hughes Medical Institute Professor. She is an elected member of the National Academy of Sciences and the American Academy of Arts and Sciences.
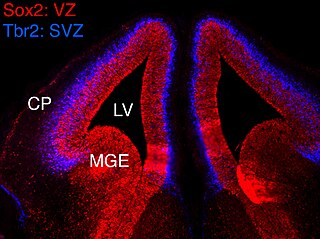
In vertebrates, the ventricular zone (VZ) is a transient embryonic layer of tissue containing neural stem cells, principally radial glial cells, of the central nervous system (CNS). The VZ is so named because it lines the ventricular system, which contains cerebrospinal fluid (CSF). The embryonic ventricular system contains growth factors and other nutrients needed for the proper function of neural stem cells. Neurogenesis, or the generation of neurons, occurs in the VZ during embryonic and fetal development as a function of the Notch pathway, and the newborn neurons must migrate substantial distances to their final destination in the developing brain or spinal cord where they will establish neural circuits. A secondary proliferative zone, the subventricular zone (SVZ), lies adjacent to the VZ. In the embryonic cerebral cortex, the SVZ contains intermediate neuronal progenitors that continue to divide into post-mitotic neurons. Through the process of neurogenesis, the parent neural stem cell pool is depleted and the VZ disappears. The balance between the rates of stem cell proliferation and neurogenesis changes during development, and species from mouse to human show large differences in the number of cell cycles, cell cycle length, and other parameters, which is thought to give rise to the large diversity in brain size and structure.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). It occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.

The Radial Unit Hypothesis (RUH) is a conceptual theory of cerebral cortex development, first described by Pasko Rakic. The RUH states that the cerebral cortex develops during embryogenesis as an array of interacting cortical columns, or 'radial units', each of which originates from a transient stem cell layer called the ventricular zone, which contains neural stem cells known as radial glial cells.
Intermediate progenitor cells (IPCs) are a type of progenitor cell in the developing cerebral cortex. They are multipolar cells produced by radial glial cells who have undergone asymmetric division. IPCs can produce neuron cells via neurogenesis and are responsible for ensuring the proper quantity of cortical neurons are produced. In mammals, neural stem cells are the primary progenitors during embryogenesis whereas intermediate progenitor cells are the secondary progenitors.
References
- ↑ notes, Dr. K. Brodmann; translated with editorial; Garey, an introduction by Laurence J. (2006). Brodmann's Localisation in the cerebral cortex : the principles of comparative localisation in the cerebral cortex based on cytoarchitectonics (3rd ed.). New York: Springer Science+Business Media. ISBN 978-0387269177.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Rakic, P (8 July 1988). "Specification of cerebral cortical areas". Science. 241 (4862): 170–6. doi:10.1126/science.3291116. PMID 3291116.
- ↑ Fukuchi-Shimogori, T; Grove, EA (2 November 2001). "Neocortex patterning by the secreted signaling molecule FGF8". Science. 294 (5544): 1071–4. doi: 10.1126/science.1064252 . PMID 11567107.
- ↑ Grove, EA; Fukuchi-Shimogori, T (2003). "Generating the cerebral cortical area map". Annual Review of Neuroscience. 26: 355–80. doi:10.1146/annurev.neuro.26.041002.131137. PMID 14527269.
- ↑ Sur, M; Rubenstein, JL (4 November 2005). "Patterning and plasticity of the cerebral cortex". Science. 310 (5749): 805–10. doi:10.1126/science.1112070. PMID 16272112.
- ↑ Ackman, JB; Burbridge, TJ; Crair, MC (11 October 2012). "Retinal waves coordinate patterned activity throughout the developing visual system". Nature. 490 (7419): 219–25. doi:10.1038/nature11529. PMC 3962269 . PMID 23060192.