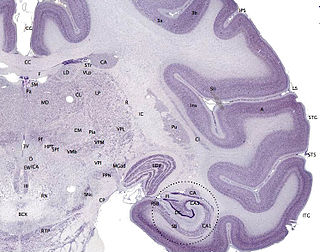
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex, with just 10% consisting of allocortex. It is separated into two cortices, by the longitudinal fissure that divides the cerebrum into the left and right cerebral hemispheres. The two hemispheres are joined beneath the cortex by the corpus callosum. The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is part of the brain responsible for cognition.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.

The neocortex, also called the neopallium, isocortex, or the six-layered cortex, is a set of layers of the mammalian cerebral cortex involved in higher-order brain functions such as sensory perception, cognition, generation of motor commands, spatial reasoning and language. The neocortex is further subdivided into the true isocortex and the proisocortex.

A cortical column is a group of neurons forming a cylindrical structure through the cerebral cortex of the brain perpendicular to the cortical surface. The structure was first identified by Mountcastle in 1957. He later identified minicolumns as the basic units of the neocortex which were arranged into columns. Each contains the same types of neurons, connectivity, and firing properties. Columns are also called hypercolumn, macrocolumn, functional column or sometimes cortical module. Neurons within a minicolumn (microcolumn) encode similar features, whereas a hypercolumn "denotes a unit containing a full set of values for any given set of receptive field parameters". A cortical module is defined as either synonymous with a hypercolumn (Mountcastle) or as a tissue block of multiple overlapping hypercolumns.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.
Joseph Altman was an American biologist who worked in the field of neurobiology.

The subventricular zone (SVZ) is a region situated on the outside wall of each lateral ventricle of the vertebrate brain. It is present in both the embryonic and adult brain. In embryonic life, the SVZ refers to a secondary proliferative zone containing neural progenitor cells, which divide to produce neurons in the process of neurogenesis. The primary neural stem cells of the brain and spinal cord, termed radial glial cells, instead reside in the ventricular zone (VZ).

Pasko Rakic is a Yugoslav-born American neuroscientist, who presently works in the Yale School of Medicine Department of Neuroscience in New Haven, Connecticut. His main research interest is in the development and evolution of the human brain. He was the founder and served as Chairman of the Department of Neurobiology at Yale, and was founder and Director of the Kavli Institute for Neuroscience. He is best known for elucidating the mechanisms involved in development and evolution of the cerebral cortex. In 2008, Rakic shared the inaugural Kavli Prize in Neuroscience. He is currently the Dorys McConell Duberg Professor of Neuroscience, leads an active research laboratory, and serves on Advisory Boards and Scientific Councils of a number of Institutions and Research Foundations.

The subgranular zone (SGZ) is a brain region in the hippocampus where adult neurogenesis occurs. The other major site of adult neurogenesis is the subventricular zone (SVZ) in the brain.

The ganglionic eminence (GE) is a transitory structure in the development of the nervous system that guides cell and axon migration. It is present in the embryonic and fetal stages of neural development found between the thalamus and caudate nucleus.
Gyrification is the process of forming the characteristic folds of the cerebral cortex.

T-box, brain, 1 is a transcription factor protein important in vertebrate embryo development. It is encoded by the TBR1 gene. This gene is also known by several other names: T-Brain 1, TBR-1, TES-56, and MGC141978. TBR1 is a member of the TBR1 subfamily of T-box family transcription factors, which share a common DNA-binding domain. Other members of the TBR1 subfamily include EOMES and TBX21. TBR1 is involved in the differentiation and migration of neurons and is required for normal brain development. TBR1 interacts with various genes and proteins in order to regulate cortical development, specifically within layer VI of the developing six-layered human cortex. Studies show that TBR1 may play a role in major neurological diseases such as Alzheimer's disease (AD), Parkinson's disease (PD) and autism spectrum disorder (ASD).
The Protomap is a primordial molecular map of the functional areas of the mammalian cerebral cortex during early embryonic development, at a stage when neural stem cells are still the dominant cell type. The protomap is a feature of the ventricular zone, which contains the principal cortical progenitor cells, known as radial glial cells. Through a process called 'cortical patterning', the protomap is patterned by a system of signaling centers in the embryo, which provide positional information and cell fate instructions. These early genetic instructions set in motion a development and maturation process that gives rise to the mature functional areas of the cortex, for example the visual, somatosensory, and motor areas. The term protomap was coined by Pasko Rakic. The protomap hypothesis was opposed by the protocortex hypothesis, which proposes that cortical proto-areas initially have the same potential, and that regionalization in large part is controlled by external influences, such as axonal inputs from the thalamus to the cortex. However, a series of papers in the year 2000 and in 2001 provided strong evidence against the protocortex hypothesis, and the protomap hypothesis has been well accepted since then. The protomap hypothesis, together with the related radial unit hypothesis, forms our core understanding of the embryonic development of the cerebral cortex. Once the basic structure is present and cortical neurons have migrated to their final destinations, many other processes contribute to the maturation of functional cortical circuits.

Eomesodermin also known as T-box brain protein 2 (Tbr2) is a protein that in humans is encoded by the EOMES gene.
Corticogenesis is the process during which the cerebral cortex of the brain is formed as part of the development of the nervous system of mammals including its development in humans. The cortex is the outer layer of the brain and is composed of up to six layers. Neurons formed in the ventricular zone migrate to their final locations in one of the six layers of the cortex. The process occurs from embryonic day 10 to 17 in mice and between gestational weeks seven to 18 in humans.
Cajal–Retzius cells are a heterogeneous population of morphologically and molecularly distinct reelin-producing cell types in the marginal zone/layer I of the developmental cerebral cortex and in the immature hippocampus of different species and at different times during embryogenesis and postnatal life.
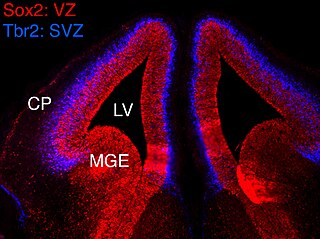
In vertebrates, the ventricular zone (VZ) is a transient embryonic layer of tissue containing neural stem cells, principally radial glial cells, of the central nervous system (CNS). The VZ is so named because it lines the ventricular system, which contains cerebrospinal fluid (CSF). The embryonic ventricular system contains growth factors and other nutrients needed for the proper function of neural stem cells. Neurogenesis, or the generation of neurons, occurs in the VZ during embryonic and fetal development as a function of the Notch pathway, and the newborn neurons must migrate substantial distances to their final destination in the developing brain or spinal cord where they will establish neural circuits. A secondary proliferative zone, the subventricular zone (SVZ), lies adjacent to the VZ. In the embryonic cerebral cortex, the SVZ contains intermediate neuronal progenitors that continue to divide into post-mitotic neurons. Through the process of neurogenesis, the parent neural stem cell pool is depleted and the VZ disappears. The balance between the rates of stem cell proliferation and neurogenesis changes during development, and species from mouse to human show large differences in the number of cell cycles, cell cycle length, and other parameters, which is thought to give rise to the large diversity in brain size and structure.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). It occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.
Cortical patterning is a field of developmental neuroscience which aims to determine how the various functional areas of the cerebral cortex are generated, what size and shape they will be, and how their spatial pattern across the surface of the cortex is specified. Early brain lesion studies indicated that different parts of the cortex served different cognitive functions, such as visual, somatosensory, and motor functions, beautifully assimilated by Brodmann in 1909. Today the field supports the idea of a 'protomap', which is a molecular pre-pattern of the cortical areas during early embryonic stages. The protomap is a feature of the cortical ventricular zone, which contains the primary stem cells of the cortex known as radial glial cells. A system of signaling centers, positioned strategically at the midline and edges of the cortex, produce secreted signaling proteins that establish concentration gradients in the cortical primordium. This provides positional information for each stem cell, and regulates proliferation, neurogenesis, and areal identity. After the initial establishment of areal identity, axons from the developing thalamus arrive at their correct cortical areal destination through the process of axon guidance and begin to form synapses. Many activity-dependent processes are then thought to play important roles in the maturation of each area.
Intermediate progenitor cells (IPCs) are a type of progenitor cell in the developing cerebral cortex. They are multipolar cells produced by radial glial cells who have undergone asymmetric division. IPCs can produce neuron cells via neurogenesis and are responsible for ensuring the proper quantity of cortical neurons are produced. In mammals, neural stem cells are the primary progenitors during embryogenesis whereas intermediate progenitor cells are the secondary progenitors.











