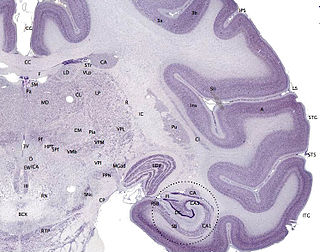
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex, with just 10% consisting of the allocortex. It is separated into two cortices, by the longitudinal fissure that divides the cerebrum into the left and right cerebral hemispheres. The two hemispheres are joined beneath the cortex by the corpus callosum. The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is part of the brain responsible for cognition.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.

Glia, also called glial cells(gliocytes) or neuroglia, are non-neuronal cells in the central nervous system (brain and spinal cord) and the peripheral nervous system that do not produce electrical impulses. The neuroglia make up more than one half the volume of neural tissue in our body. They maintain homeostasis, form myelin in the peripheral nervous system, and provide support and protection for neurons. In the central nervous system, glial cells include oligodendrocytes, astrocytes, ependymal cells and microglia, and in the peripheral nervous system they include Schwann cells and satellite cells.
In vertebrates, a neuroblast or primitive nerve cell is a postmitotic cell that does not divide further, and which will develop into a neuron after a migration phase. In invertebrates such as Drosophila, neuroblasts are neural progenitor cells which divide asymmetrically to produce a neuroblast, and a daughter cell of varying potency depending on the type of neuroblast. Vertebrate neuroblasts differentiate from radial glial cells and are committed to becoming neurons. Neural stem cells, which only divide symmetrically to produce more neural stem cells, transition gradually into radial glial cells. Radial glial cells, also called radial glial progenitor cells, divide asymmetrically to produce a neuroblast and another radial glial cell that will re-enter the cell cycle.

Astrocytes, also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, regulation of cerebral blood flow, and a role in the repair and scarring process of the brain and spinal cord following infection and traumatic injuries. The proportion of astrocytes in the brain is not well defined; depending on the counting technique used, studies have found that the astrocyte proportion varies by region and ranges from 20% to around 40% of all glia. Another study reports that astrocytes are the most numerous cell type in the brain. Astrocytes are the major source of cholesterol in the central nervous system. Apolipoprotein E transports cholesterol from astrocytes to neurons and other glial cells, regulating cell signaling in the brain. Astrocytes in humans are more than twenty times larger than in rodent brains, and make contact with more than ten times the number of synapses.
Oligodendrocyte progenitor cells (OPCs), also known as oligodendrocyte precursor cells, NG2-glia, O2A cells, or polydendrocytes, are a subtype of glia in the central nervous system named for their essential role as precursors to oligodendrocytes. They are typically identified in the human by co-expression of PDGFRA and CSPG4.
Neural stem cells (NSCs) are self-renewing, multipotent cells that firstly generate the radial glial progenitor cells that generate the neurons and glia of the nervous system of all animals during embryonic development. Some neural progenitor stem cells persist in highly restricted regions in the adult vertebrate brain and continue to produce neurons throughout life. Differences in the size of the central nervous system are among the most important distinctions between the species and thus mutations in the genes that regulate the size of the neural stem cell compartment are among the most important drivers of vertebrate evolution.

The subventricular zone (SVZ) is a region situated on the outside wall of each lateral ventricle of the vertebrate brain. It is present in both the embryonic and adult brain. In embryonic life, the SVZ refers to a secondary proliferative zone containing neural progenitor cells, which divide to produce neurons in the process of neurogenesis. The primary neural stem cells of the brain and spinal cord, termed radial glial cells, instead reside in the ventricular zone (VZ).

Müller glia, or Müller cells, are a type of retinal glial cells, first recognized and described by Heinrich Müller. They are found in the vertebrate retina, where they serve as support cells for the neurons, as all glial cells do. They are the most common type of glial cell found in the retina. While their cell bodies are located in the inner nuclear layer of the retina, they span across the entire retina.

The subgranular zone (SGZ) is a brain region in the hippocampus where adult neurogenesis occurs. The other major site of adult neurogenesis is the subventricular zone (SVZ) in the brain.
Gyrification is the process of forming the characteristic folds of the cerebral cortex.

Eomesodermin also known as T-box brain protein 2 (Tbr2) is a protein that in humans is encoded by the EOMES gene.
Endogenous regeneration in the brain is the ability of cells to engage in the repair and regeneration process. While the brain has a limited capacity for regeneration, endogenous neural stem cells, as well as numerous pro-regenerative molecules, can participate in replacing and repairing damaged or diseased neurons and glial cells. Another benefit that can be achieved by using endogenous regeneration could be avoiding an immune response from the host.
The development of the cerebral cortex, known as corticogenesis is the process during which the cerebral cortex of the brain is formed as part of the development of the nervous system of mammals including its development in humans. The cortex is the outer layer of the brain and is composed of up to six layers. Neurons formed in the ventricular zone migrate to their final locations in one of the six layers of the cortex. The process occurs from embryonic day 10 to 17 in mice and between gestational weeks seven to 18 in humans.
Proneural genes encode transcription factors of the basic helix-loop-helix (bHLH) class which are responsible for the development of neuroectodermal progenitor cells. Proneural genes have multiple functions in neural development. They integrate positional information and contribute to the specification of progenitor-cell identity. From the same ectodermal cell types, neural or epidermal cells can develop based on interactions between proneural and neurogenic genes. Neurogenic genes are so called because loss of function mutants show an increase number of developed neural precursors. On the other hand, proneural genes mutants fail to develop neural precursor cells.

A neuronal lineage marker is an endogenous tag that is expressed in different cells along neurogenesis and differentiated cells such as neurons. It allows detection and identification of cells by using different techniques. A neuronal lineage marker can be either DNA, mRNA or RNA expressed in a cell of interest. It can also be a protein tag, as a partial protein, a protein or an epitope that discriminates between different cell types or different states of a common cell. An ideal marker is specific to a given cell type in normal conditions and/or during injury. Cell markers are very valuable tools for examining the function of cells in normal conditions as well as during disease. The discovery of various proteins specific to certain cells led to the production of cell-type-specific antibodies that have been used to identify cells.
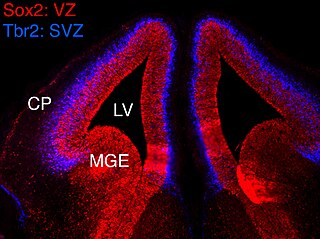
In vertebrates, the ventricular zone (VZ) is a transient embryonic layer of tissue containing neural stem cells, principally radial glial cells, of the central nervous system (CNS). The VZ is so named because it lines the ventricular system, which contains cerebrospinal fluid (CSF). The embryonic ventricular system contains growth factors and other nutrients needed for the proper function of neural stem cells. Neurogenesis, or the generation of neurons, occurs in the VZ during embryonic and fetal development as a function of the Notch pathway, and the newborn neurons must migrate substantial distances to their final destination in the developing brain or spinal cord where they will establish neural circuits. A secondary proliferative zone, the subventricular zone (SVZ), lies adjacent to the VZ. In the embryonic cerebral cortex, the SVZ contains intermediate neuronal progenitors that continue to divide into post-mitotic neurons. Through the process of neurogenesis, the parent neural stem cell pool is depleted and the VZ disappears. The balance between the rates of stem cell proliferation and neurogenesis changes during development, and species from mouse to human show large differences in the number of cell cycles, cell cycle length, and other parameters, which is thought to give rise to the large diversity in brain size and structure.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). In short, it is brain growth in relation to its organization. This occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.
Intermediate progenitor cells (IPCs) are a type of progenitor cell in the developing cerebral cortex. They are multipolar cells produced by radial glial cells who have undergone asymmetric division. IPCs can produce neuron cells via neurogenesis and are responsible for ensuring the proper quantity of cortical neurons are produced. In mammals, neural stem cells are the primary progenitors during embryogenesis whereas intermediate progenitor cells are the secondary progenitors.
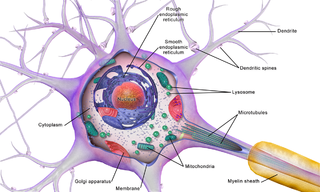
Brain cells make up the functional tissue of the brain. The rest of the brain tissue is structural or connective called the stroma which includes blood vessels. The two main types of cells in the brain are neurons, also known as nerve cells, and glial cells, also known as neuroglia.













