
The cerebellum is a major feature of the hindbrain of all vertebrates. Although usually smaller than the cerebrum, in some animals such as the mormyrid fishes it may be as large as it or even larger. In humans, the cerebellum plays an important role in motor control. It may also be involved in some cognitive functions such as attention and language as well as emotional control such as regulating fear and pleasure responses, but its movement-related functions are the most solidly established. The human cerebellum does not initiate movement, but contributes to coordination, precision, and accurate timing: it receives input from sensory systems of the spinal cord and from other parts of the brain, and integrates these inputs to fine-tune motor activity. Cerebellar damage produces disorders in fine movement, equilibrium, posture, and motor learning in humans.
The dentate gyrus (DG) is part of the hippocampal formation in the temporal lobe of the brain, which also includes the hippocampus and the subiculum. The dentate gyrus is part of the hippocampal trisynaptic circuit and is thought to contribute to the formation of new episodic memories, the spontaneous exploration of novel environments and other functions.

The olfactory bulb is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OFC) and the hippocampus where it plays a role in emotion, memory and learning. The bulb is divided into two distinct structures: the main olfactory bulb and the accessory olfactory bulb. The main olfactory bulb connects to the amygdala via the piriform cortex of the primary olfactory cortex and directly projects from the main olfactory bulb to specific amygdala areas. The accessory olfactory bulb resides on the dorsal-posterior region of the main olfactory bulb and forms a parallel pathway. Destruction of the olfactory bulb results in ipsilateral anosmia, while irritative lesions of the uncus can result in olfactory and gustatory hallucinations.
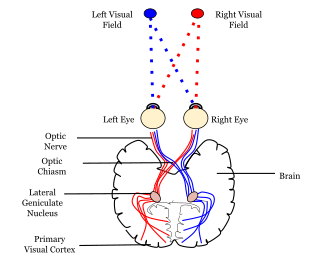
In neuroanatomy, a neural pathway is the connection formed by axons that project from neurons to make synapses onto neurons in another location, to enable neurotransmission. Neurons are connected by a single axon, or by a bundle of axons known as a nerve tract, or fasciculus. Shorter neural pathways are found within grey matter in the brain, whereas longer projections, made up of myelinated axons, constitute white matter.

Pyramidal cells, or pyramidal neurons, are a type of multipolar neuron found in areas of the brain including the cerebral cortex, the hippocampus, and the amygdala. Pyramidal cells are the primary excitation units of the mammalian prefrontal cortex and the corticospinal tract. One of the main structural features of the pyramidal neuron is the conic shaped soma, or cell body, after which the neuron is named. Other key structural features of the pyramidal cell are a single axon, a large apical dendrite, multiple basal dendrites, and the presence of dendritic spines.

Purkinje cells or Purkinje neurons, named for Czech physiologist Jan Evangelista Purkyně who identified them in 1837, are a unique type of prominent large neurons located in the cerebellar cortex of the brain. With their flask-shaped cell bodies, many branching dendrites, and a single long axon, these cells are essential for controlling motor activity. Purkinje cells mainly release GABA neurotransmitter, which inhibits some neurons to reduce nerve impulse transmission. Purkinje cells efficiently control and coordinate the body's motor motions through these inhibitory actions.
An apical dendrite is a dendrite that emerges from the apex of a pyramidal cell. Apical dendrites are one of two primary categories of dendrites, and they distinguish the pyramidal cells from spiny stellate cells in the cortices. Pyramidal cells are found in the prefrontal cortex, the hippocampus, the entorhinal cortex, the olfactory cortex, and other areas. Dendrite arbors formed by apical dendrites are the means by which synaptic inputs into a cell are integrated. The apical dendrites in these regions contribute significantly to memory, learning, and sensory associations by modulating the excitatory and inhibitory signals received by the pyramidal cells.

In neuroscience, Golgi cells are the most abundant inhibitory interneurons found within the granular layer of the cerebellum. Golgi cells can be found in the granular layer at various layers. The Golgi cell is essential for controlling the activity of the granular layer. They were first identified as inhibitory in 1964. It was also the first example of an inhibitory feedback network in which the inhibitory interneuron was identified anatomically. Golgi cells produce a wide lateral inhibition that reaches beyond the afferent synaptic field and inhibit granule cells via feedforward and feedback inhibitory loops. These cells synapse onto the dendrite of granule cells and unipolar brush cells. They receive excitatory input from mossy fibres, also synapsing on granule cells, and parallel fibers, which are long granule cell axons. Thereby this circuitry allows for feed-forward and feed-back inhibition of granule cells.

Mitral cells are neurons that are part of the olfactory system. They are located in the olfactory bulb in the mammalian central nervous system. They receive information from the axons of olfactory receptor neurons, forming synapses in neuropils called glomeruli. Axons of the mitral cells transfer information to a number of areas in the brain, including the piriform cortex, entorhinal cortex, and amygdala. Mitral cells receive excitatory input from olfactory sensory neurons and external tufted cells on their primary dendrites, whereas inhibitory input arises either from granule cells onto their lateral dendrites and soma or from periglomerular cells onto their dendritic tuft. Mitral cells together with tufted cells form an obligatory relay for all olfactory information entering from the olfactory nerve. Mitral cell output is not a passive reflection of their input from the olfactory nerve. In mice, each mitral cell sends a single primary dendrite into a glomerulus receiving input from a population of olfactory sensory neurons expressing identical olfactory receptor proteins, yet the odor responsiveness of the 20-40 mitral cells connected to a single glomerulus is not identical to the tuning curve of the input cells, and also differs between sister mitral cells. Odorant response properties of individual neurons in an olfactory glomerular module. The exact type of processing that mitral cells perform with their inputs is still a matter of controversy. One prominent hypothesis is that mitral cells encode the strength of an olfactory input into their firing phases relative to the sniff cycle. A second hypothesis is that the olfactory bulb network acts as a dynamical system that decorrelates to differentiate between representations of highly similar odorants over time. Support for the second hypothesis comes primarily from research in zebrafish.
The stratum lucidum of the hippocampus is a layer of the hippocampus between the stratum pyramidale and the stratum radiatum. It is the tract of the mossy fiber projections, both inhibitory and excitatory from the granule cells of the dentate gyrus. One mossy fiber may make up to 37 connections to a single pyramidal cell, and innervate around 12 pyramidal cells on top of that. Any given pyramidal cell in the stratum lucidum may get input from as many as 50 granule cells.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance. The axons that make up the pathway emerge from the basal portions of the granule cells and pass through the hilus of the dentate gyrus before entering the stratum lucidum of CA3. Granule cell synapses tend to be glutamatergic, though immunohistological data has indicated that some synapses contain neuropeptidergic elements including opiate peptides such as dynorphin and enkephalin. There is also evidence for co-localization of both GABAergic and glutamatergic neurotransmitters within mossy fiber terminals. GABAergic and glutamatergic co-localization in mossy fiber boutons has been observed primarily in the developing hippocampus, but in adulthood, evidence suggests that mossy fiber synapses may alternate which neurotransmitter is released through activity-dependent regulation.

Mossy fibers are one of the major inputs to cerebellum. There are many sources of this pathway, the largest of which is the cerebral cortex, which sends input to the cerebellum via the pontocerebellar pathway. Other contributors include the vestibular nerve and nuclei, the spinal cord, the reticular formation, and feedback from deep cerebellar nuclei. Axons enter the cerebellum via the middle and inferior cerebellar peduncles, where some branch to make contact with deep cerebellar nuclei. They ascend into the white matter of the cerebellum, where each axon branches to innervate granule cells in several cerebellar folia.

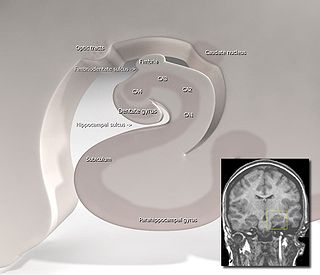
Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In primate brains, including humans, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.

The anatomy of the cerebellum can be viewed at three levels. At the level of gross anatomy, the cerebellum consists of a tightly folded and crumpled layer of cortex, with white matter underneath, several deep nuclei embedded in the white matter, and a fluid-filled ventricle in the middle. At the intermediate level, the cerebellum and its auxiliary structures can be broken down into several hundred or thousand independently functioning modules or compartments known as microzones. At the microscopic level, each module consists of the same small set of neuronal elements, laid out with a highly stereotyped geometry.

Cerebellar granule cells form the thick granular layer of the cerebellar cortex and are among the smallest neurons in the brain. Cerebellar granule cells are also the most numerous neurons in the brain: in humans, estimates of their total number average around 50 billion, which means that they constitute about 3/4 of the brain's neurons.
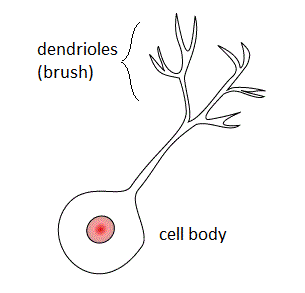
Unipolar brush cells (UBCs) are a class of excitatory glutamatergic interneuron found in the granular layer of the cerebellar cortex and also in the granule cell domain of the cochlear nucleus.

The cerebellar glomerulus is a small, intertwined mass of nerve fiber terminals in the granular layer of the cerebellar cortex. It consists of post-synaptic granule cell dendrites and pre-synaptic terminals of mossy fibers.
Dendrodendritic synapses are connections between the dendrites of two different neurons. This is in contrast to the more common axodendritic synapse (chemical synapse) where the axon sends signals and the dendrite receives them. Dendrodendritic synapses are activated in a similar fashion to axodendritic synapses in respects to using a chemical synapse. An incoming action potential permits the release of neurotransmitters to propagate the signal to the post synaptic cell. There is evidence that these synapses are bi-directional, in that either dendrite can signal at that synapse. Ordinarily, one of the dendrites will display inhibitory effects while the other will display excitatory effects. The actual signaling mechanism utilizes Na+ and Ca2+ pumps in a similar manner to those found in axodendritic synapses.
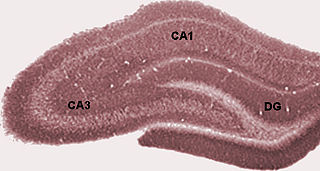
The hippocampus proper refers to the actual structure of the hippocampus which is made up of three regions or subfields. The subfields CA1, CA2, and CA3 use the initials of cornu Ammonis, an earlier name of the hippocampus.
An axo-axonic synapse is a type of synapse, formed by one neuron projecting its axon terminals onto another neuron's axon.















