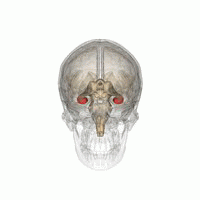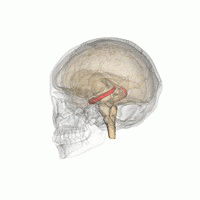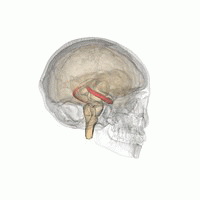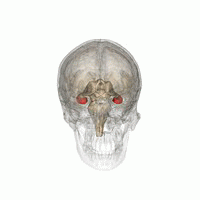
The hippocampus is a major component of the brain of humans and other vertebrates. Humans and other mammals have two hippocampi, one in each side of the brain. The hippocampus is part of the limbic system, and plays important roles in the consolidation of information from short-term memory to long-term memory, and in spatial memory that enables navigation. The hippocampus is located in the allocortex, with neural projections into the neocortex, in humans as well as other primates. The hippocampus, as the medial pallium, is a structure found in all vertebrates. In humans, it contains two main interlocking parts: the hippocampus proper, and the dentate gyrus.

The olfactory bulb is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OFC) and the hippocampus where it plays a role in emotion, memory and learning. The bulb is divided into two distinct structures: the main olfactory bulb and the accessory olfactory bulb. The main olfactory bulb connects to the amygdala via the piriform cortex of the primary olfactory cortex and directly projects from the main olfactory bulb to specific amygdala areas. The accessory olfactory bulb resides on the dorsal-posterior region of the main olfactory bulb and forms a parallel pathway. Destruction of the olfactory bulb results in ipsilateral anosmia, while irritative lesions of the uncus can result in olfactory and gustatory hallucinations.

Adult neurogenesis is the process in which neurons are generated from neural stem cells in the adult. This process differs from prenatal neurogenesis.
Schaffer collaterals are axon collaterals given off by CA3 pyramidal cells in the hippocampus. These collaterals project to area CA1 of the hippocampus and are an integral part of memory formation and the emotional network of the Papez circuit, and of the hippocampal trisynaptic loop. It is one of the most studied synapses in the world and named after the Hungarian anatomist-neurologist Károly Schaffer.
In the brain, the perforant path or perforant pathway provides a connectional route from the entorhinal cortex to all fields of the hippocampal formation, including the dentate gyrus, all CA fields, and the subiculum.
Elizabeth Gould is an American neuroscientist and the Dorman T. Warren Professor of Psychology at Princeton University. She was an early investigator of adult neurogenesis in the hippocampus, a research area that continues to be controversial. In November 2002, Discover magazine listed her as one of the 50 most important women scientists.
The stratum lucidum of the hippocampus is a layer of the hippocampus between the stratum pyramidale and the stratum radiatum. It is the tract of the mossy fiber projections, both inhibitory and excitatory from the granule cells of the dentate gyrus. One mossy fiber may make up to 37 connections to a single pyramidal cell, and innervate around 12 pyramidal cells on top of that. Any given pyramidal cell in the stratum lucidum may get input from as many as 50 granule cells.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance. The axons that make up the pathway emerge from the basal portions of the granule cells and pass through the hilus of the dentate gyrus before entering the stratum lucidum of CA3. Granule cell synapses tend to be glutamatergic, though immunohistological data has indicated that some synapses contain neuropeptidergic elements including opiate peptides such as dynorphin and enkephalin. There is also evidence for co-localization of both GABAergic and glutamatergic neurotransmitters within mossy fiber terminals. GABAergic and glutamatergic co-localization in mossy fiber boutons has been observed primarily in the developing hippocampus, but in adulthood, evidence suggests that mossy fiber synapses may alternate which neurotransmitter is released through activity-dependent regulation.

The subgranular zone (SGZ) is a brain region in the hippocampus where adult neurogenesis occurs. The other major site of adult neurogenesis is the subventricular zone (SVZ) in the brain.
The trisynaptic circuit, or trisynaptic loop is a relay of synaptic transmission in the hippocampus. The circuit was initially described by the neuroanatomist Santiago Ramon y Cajal, in the early twentieth century, using the Golgi staining method. After the discovery of the trisynaptic circuit, a series of research has been conducted to determine the mechanisms driving this circuit. Today, research is focused on how this loop interacts with other parts of the brain, and how it influences human physiology and behaviour. For example, it has been shown that disruptions within the trisynaptic circuit lead to behavioural changes in rodent and feline models.

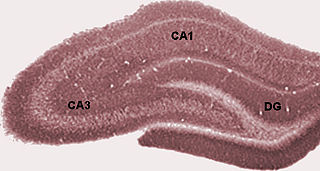
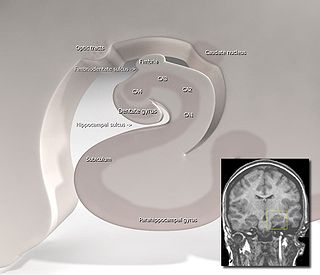
Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In primate brains, including humans, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.

The fascia dentata is the earliest stage of the hippocampal circuit. Its primary input is the perforant path from the superficial layers of entorhinal cortex. Its principal neurons are tiny granule cells which give rise to unmyelinated axons called the mossy fibers which project to the hilus and CA3. The fascia dentata of the rat contains approximately 1,000,000 granule cells. It receives feedback connections from mossy cells in the hilus at distant levels in the septal and temporal directions. The fascia dentata and the hilus together make up the dentate gyrus. As with all regions of the hippocampus, the dentate gyrus also receives GABAergic and cholinergic input from the medial septum and the diagonal band of Broca.

The name granule cell has been used for a number of different types of neurons whose only common feature is that they all have very small cell bodies. Granule cells are found within the granular layer of the cerebellum, the dentate gyrus of the hippocampus, the superficial layer of the dorsal cochlear nucleus, the olfactory bulb, and the cerebral cortex.
Endogenous regeneration in the brain is the ability of cells to engage in the repair and regeneration process. While the brain has a limited capacity for regeneration, endogenous neural stem cells, as well as numerous pro-regenerative molecules, can participate in replacing and repairing damaged or diseased neurons and glial cells. Another benefit that can be achieved by using endogenous regeneration could be avoiding an immune response from the host.

The hippocampus is an area of the brain integral to learning and memory. Removal of this structure can result in the inability to form new memories as most famously demonstrated in a patient referred to as HM. The unique morphology of the hippocampus can be appreciated without the use of special stains and this distinct circuitry has helped further the understanding of neuronal signal potentiation. The following will provide an introduction to hippocampal development with particular focus on the role of glucocorticoid signaling.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). In short, it is brain growth in relation to its organization. This occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.
Attila Losonczy is a Hungarian neuroscientist, Professor of Neuroscience at Columbia University Medical Center. Losonczy's main area of research is on the relationship between neural networks and behavior, specifically with regard to learning in the hippocampus.
The hippocampus proper refers to the actual structure of the hippocampus which is made up of three regions or subfields. The subfields CA1, CA2, and CA3 use the initials of cornu Ammonis, an earlier name of the hippocampus.
The supramammillary nucleus (SuM), or supramammillary area, is a thin layer of cells in the brain that lies above the mammillary bodies. It can be considered part of the hypothalamus and diencephalon. The nucleus can be divided into medial and lateral sections. The medial SuM, or SuMM, is made of smaller cells which release dopamine and give input to the lateral septal nucleus. The lateral SuM, or SuML, is made of larger cells that project to the hippocampus.

Adult neurogenesis is the process by which functional, mature neurons are produced from neural stem cells (NSCs) in the adult brain. In most mammals, including humans, it only occurs in the subgranular zone of the hippocampus, and in the olfactory bulb. The neurogenesis hypothesis of depression proposes that major depressive disorder is caused, at least partly, by impaired neurogenesis in the subgranular zone of the hippocampus.














