
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

Health informatics is the study and implementation of computer structures and algorithms to improve communication, understanding, and management of medical information. It can be viewed as branch of engineering and applied science.
A hospital information system (HIS) is an element of health informatics that focuses mainly on the administrational needs of hospitals. In many implementations, a HIS is a comprehensive, integrated information system designed to manage all the aspects of a hospital's operation, such as medical, administrative, financial, and legal issues and the corresponding processing of services. Hospital information system is also known as hospital management software (HMS) or hospital management system.
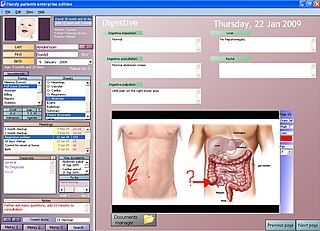
An electronic health record (EHR) is the systematized collection of patient and population electronically stored health information in a digital format. These records can be shared across different health care settings. Records are shared through network-connected, enterprise-wide information systems or other information networks and exchanges. EHRs may include a range of data, including demographics, medical history, medication and allergies, immunization status, laboratory test results, radiology images, vital signs, personal statistics like age and weight, and billing information.

Hill-Rom Holdings, Inc., doing business as Hillrom, is an American medical technology provider that is a subsidiary of Baxter International.
A Clinical Trial Management System (CTMS) is a software system used by biotechnology and pharmaceutical industries to manage clinical trials in clinical research. The system maintains and manages planning, performing and reporting functions, along with participant contact information, tracking deadlines and milestones.
A Clinical Research Coordinator (CRC) is a person responsible for conducting clinical trials using good clinical practice (GCP) under the auspices of a Principal Investigator (PI).
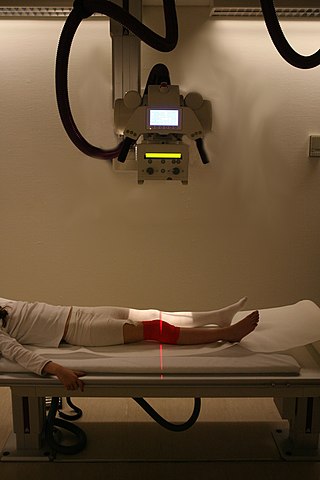
Imaging informatics, also known as radiology informatics or medical imaging informatics, is a subspecialty of biomedical informatics that aims to improve the efficiency, accuracy, usability and reliability of medical imaging services within the healthcare enterprise. It is devoted to the study of how information about and contained within medical images is retrieved, analyzed, enhanced, and exchanged throughout the medical enterprise.
Medical diagnosis is the process of determining which disease or condition explains a person's symptoms and signs. It is most often referred to as a diagnosis with the medical context being implicit. The information required for a diagnosis is typically collected from a history and physical examination of the person seeking medical care. Often, one or more diagnostic procedures, such as medical tests, are also done during the process. Sometimes the posthumous diagnosis is considered a kind of medical diagnosis.
FotoFinder is a worldwide brand for medical skin imaging systems. The German company FotoFinder Systems GmbH was founded in 1991 and has developed imaging solutions for the follow-up of skin lesions as well as hair disorders diagnostics (TrichoLAB) and psoriasis assessment.
Patient recruitment is the process of finding and enrolling suitable participants for clinical trials. It is a crucial aspect of drug development and medical research, as it affects the validity, reliability, and generalizability of the results. Patient recruitment can also be challenging, time-consuming, and costly, involving various ethical, regulatory, and logistical issues.
ePharmaSolutions (ePS), as a contract research organization, is an American clinical research provider. The solutions include proprietary software applications and global clinical services that focus on the major areas of clinical study delays.
A clinical trial portal is a web portal or enterprise portal that primarily serves sponsors and investigators in a clinical trial. Clinical portals can be developed for a particular study, however study-specific portals may be part of larger, clinical sponsor or Contract Research Organization (CRO) portals that cover multiple trials. A clinical portal is typically developed by a sponsor or CRO to facilitate centralized access to relevant information, documentation and online applications by investigational sites participating in a trial, as well as for the monitors, study managers, data managers, medical, safety and regulatory staff that help plan, conduct, manage and review the trial.

In medicine, monitoring is the observation of a disease, condition or one or several medical parameters over time.

Remote patient monitoring (RPM) is a technology to enable monitoring of patients outside of conventional clinical settings, such as in the home or in a remote area, which may increase access to care and decrease healthcare delivery costs. RPM involves the constant remote care of patients by their physicians, often to track physical symptoms, chronic conditions, or post-hospitalization rehab.
Clinical point of care (POC) is the point in time when clinicians deliver healthcare products and services to patients at the time of care.
Healthcare CRM, also known as Healthcare Relationship Management, is a broadly used term for a Customer relationship management system, or CRM, used in healthcare.
RLDatix is a global enterprise software company offering software and services tailored to healthcare organizations. The technology platform is designed to support hospitals and other providers with risk mitigation, regulatory compliance, and workforce management resources.
Digital therapeutics, a subset of digital health, are evidence-based therapeutic interventions driven by high quality software programs to prevent, manage, or treat a medical disorder or disease. Digital therapeutic companies should publish trial results inclusive of clinically meaningful outcomes in peer-reviewed journals. The treatment relies on behavioral and lifestyle changes usually spurred by a collection of digital impetuses. Because of the digital nature of the methodology, data can be collected and analyzed as both a progress report and a preventative measure. Treatments are being developed for the prevention and management of a wide variety of diseases and conditions, including type 1 & type II diabetes, congestive heart failure, obesity, Alzheimer's disease, dementia, asthma, substance abuse, ADHD, hypertension, anxiety, depression, and several others. Digital therapeutics often employ strategies rooted in cognitive behavioral therapy.

Applied Spectral Imaging or ASI is a multinational biomedical company that develops and manufactures microscopy imaging and digital analysis tools for hospitals, service laboratories and research centers. The company provides cytogenetic, pathology, and research laboratories with bright-field, fluorescence and spectral imaging in clinical applications. Test slides can be scanned, captured, archived, reviewed on the screen, analyzed with computer-assisted algorithms, and reported. ASI system platforms automate the workflow process to reduce human error in the identification and classification of chromosomal disorders, genome instability, various oncological malignancies, among other diseases.







