
Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, then, are metastases (mets). It is generally distinguished from cancer invasion, which is the direct extension and penetration by cancer cells into neighboring tissues.

Small-cell carcinoma is a type of highly malignant cancer that most commonly arises within the lung, although it can occasionally arise in other body sites, such as the cervix, prostate, and gastrointestinal tract. Compared to non-small cell carcinoma, small cell carcinoma is more aggressive, with a shorter doubling time, higher growth fraction, and earlier development of metastases.

Invasive carcinoma of no special type, invasive breast carcinoma of no special type (IBC-NST), invasive ductal carcinoma (IDC), infiltrating ductal carcinoma (IDC) or invasive ductal carcinoma, not otherwise specified (NOS) is a disease. For international audiences this article will use "invasive carcinoma NST" because it is the preferred term of the World Health Organization (WHO).
Adjuvant therapy, also known as adjunct therapy, adjuvant care, or augmentation therapy, is a therapy that is given in addition to the primary or initial therapy to maximize its effectiveness. The surgeries and complex treatment regimens used in cancer therapy have led the term to be used mainly to describe adjuvant cancer treatments. An example of such adjuvant therapy is the additional treatment usually given after surgery where all detectable disease has been removed, but where there remains a statistical risk of relapse due to the presence of undetected disease. If known disease is left behind following surgery, then further treatment is not technically adjuvant.
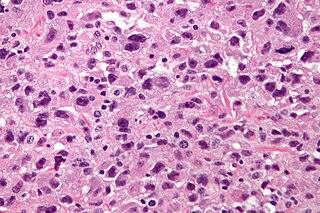
Undifferentiated pleomorphic sarcoma (UPS), also termed pleomorphic myofibrosarcoma, high-grade myofibroblastic sarcoma, and high-grade myofibrosarcoma, is characterized by the World Health Organization (WHO), 2020, as a rare, poorly differentiated neoplasm, i.e. an abnormal growth of cells that have an unclear identity and/or cell of origin. WHO classified it as one of the undifferentiated/unclassified sarcomas in the category of tumors of uncertain differentiation. Sarcomas are cancers known or thought to derive from mesenchymal stem cells that typically develop in bone, muscle, fat, blood vessels, lymphatic vessels, tendons, and ligaments. More than 70 sarcoma subtypes have been described. The UPS subtype of these sarcomas consists of tumor cells that are poorly differentiated and may appear as spindle-shaped cells, histiocytes, and giant cells. UPS is considered a diagnosis that defies formal sub-classification after thorough histologic, immunohistochemical, and ultrastructural examinations fail to identify the type of cells involved.
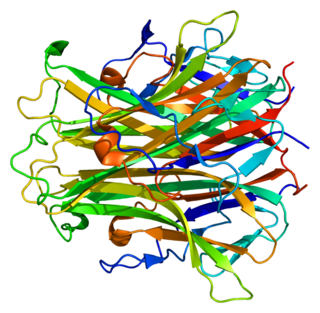
Receptor activator of nuclear factor kappa-Β ligand (RANKL), also known as tumor necrosis factor ligand superfamily member 11 (TNFSF11), TNF-related activation-induced cytokine (TRANCE), osteoprotegerin ligand (OPGL), and osteoclast differentiation factor (ODF), is a protein that in humans is encoded by the TNFSF11 gene.
Triple-negative breast cancer (TNBC) is any breast cancer that either lacks or shows low levels of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) overexpression and/or gene amplification. Triple-negative is sometimes used as a surrogate term for basal-like.

72 kDa type IV collagenase also known as matrix metalloproteinase-2 (MMP-2) and gelatinase A is an enzyme that in humans is encoded by the MMP2 gene. The MMP2 gene is located on chromosome 16 at position 12.2.
Breast cancer management takes different approaches depending on physical and biological characteristics of the disease, as well as the age, over-all health and personal preferences of the patient. Treatment types can be classified into local therapy and systemic treatment. Local therapy is most efficacious in early stage breast cancer, while systemic therapy is generally justified in advanced and metastatic disease, or in diseases with specific phenotypes.
A metastasis suppressor is a protein that acts to slow or prevent metastases from spreading in the body of an organism with cancer. Metastasis is one of the most lethal cancer processes. This process is responsible for about ninety percent of human cancer deaths. Proteins that act to slow or prevent metastases are different from those that act to suppress tumor growth. Genes for about a dozen such proteins are known in humans and other animals.

Leptomeningeal cancer is a rare complication of cancer in which the disease spreads from the original tumor site to the meninges surrounding the brain and spinal cord. This leads to an inflammatory response, hence the alternative names neoplastic meningitis (NM), malignant meningitis, or carcinomatous meningitis. The term leptomeningeal describes the thin meninges, the arachnoid and the pia mater, between which the cerebrospinal fluid is located. The disorder was originally reported by Eberth in 1870. It is also known as leptomeningeal carcinomatosis, leptomeningeal disease (LMD), leptomeningeal metastasis, meningeal metastasis and meningeal carcinomatosis.

Invadopodia are actin-rich protrusions of the plasma membrane that are associated with degradation of the extracellular matrix in cancer invasiveness and metastasis. Very similar to podosomes, invadopodia are found in invasive cancer cells and are important for their ability to invade through the extracellular matrix, especially in cancer cell extravasation. Invadopodia are generally visualized by the holes they create in ECM -coated plates, in combination with immunohistochemistry for the invadopodia localizing proteins such as cortactin, actin, Tks5 etc. Invadopodia can also be used as a marker to quantify the invasiveness of cancer cell lines in vitro using a hyaluronic acid hydrogel assay.

Bone metastasis, or osseous metastatic disease, is a category of cancer metastases that result from primary tumor invasions into bones. Bone-originating primary tumors such as osteosarcoma, chondrosarcoma, and Ewing sarcoma are rare; the most common bone tumor is a metastasis. Bone metastases can be classified as osteolytic, osteoblastic, or both. Unlike hematologic malignancies which originate in the blood and form non-solid tumors, bone metastases generally arise from epithelial tumors and form a solid mass inside the bone. Bone metastases, especially in a state of advanced disease, can cause severe pain, characterized by a dull, constant ache with periodic spikes of incident pain.

A brain metastasis is a cancer that has metastasized (spread) to the brain from another location in the body and is therefore considered a secondary brain tumor. The metastasis typically shares a cancer cell type with the original site of the cancer. Metastasis is the most common cause of brain cancer, as primary tumors that originate in the brain are less common. The most common sites of primary cancer which metastasize to the brain are lung, breast, colon, kidney, and skin cancer. Brain metastases can occur in patients months or even years after their original cancer is treated. Brain metastases have a poor prognosis for cure, but modern treatments are allowing patients to live months and sometimes years after the diagnosis.
Neuro-oncology is the study of brain and spinal cord neoplasms, many of which are very dangerous and life-threatening. Among the malignant brain cancers, gliomas of the brainstem and pons, glioblastoma multiforme, and high-grade astrocytoma/oligodendroglioma are among the worst. In these cases, untreated survival usually amounts to only a few months, and survival with current radiation and chemotherapy treatments may extend that time from around a year to a year and a half, possibly two or more, depending on the patient's condition, immune function, treatments used, and the specific type of malignant brain neoplasm. Surgery may in some cases be curative, but, as a general rule, malignant brain cancers tend to regenerate and emerge from remission easily, especially highly malignant cases. In such cases, the goal is to excise as much of the mass and as much of the tumor margin as possible without endangering vital functions or other important cognitive abilities. The Journal of Neuro-Oncology is the longest continuously published journal in the field and serves as a leading reference to those practicing in the area of neuro-oncology.

Vasculogenic mimicry (VM) is a strategy used by tumors to ensure sufficient blood supply is brought to its cells through establishing new tumor vascularization. This process is similar to tumor angiogenesis; on the other hand vascular mimicry is unique in that this process occurs independent of endothelial cells. Vasculature is instead developed de novo by cancer cells, which under stress conditions such as hypoxia, express similar properties to stem cells, capable of differentiating to mimic the function of endothelial cells and form vasculature-like structures. The ability of tumors to develop and harness nearby vasculature is considered one of the hallmarks of cancer disease development and is thought to be closely linked to tumor invasion and metastasis. Vascular mimicry has been observed predominantly in aggressive and metastatic cancers and has been associated with negative tumor characteristics such as increased metastasis, increased tissue invasion, and overall poor outcomes for patient survival. Vascular mimicry poses a serious problem for current therapeutic strategies due to its ability to function in the presence of Anti-angiogenic therapeutic agents. In fact, such therapeutics have been found to actually drive VM formation in tumors, causing more aggressive and difficult to treat tumors to develop.
A pre-metastatic niche is an environment in a secondary organ that can be conducive to the metastasis of a primary tumor. Such a niche provides favorable conditions for growth, and eventual metastasis, in an otherwise foreign and hostile environment for the primary tumor cells. This concept demonstrated the fundamental role of the microenvironment in regulating tumor growth and metastasis. The discovery of the pre-metastatic niche has fostered new research regarding the potential treatment of metastases, including targeting myeloid derived suppressor cells, and stromal cell plasticity including fibroblasts and pericytes and perivascular smooth muscle cells and (attempts to stop the flow of vesicles from primary tumors to pre-metastatic niches in secondary organs and different combinations of microenvironment targeted therapies.

Invasion is the process by which cancer cells directly extend and penetrate into neighboring tissues in cancer. It is generally distinguished from metastasis, which is the spread of cancer cells through the circulatory system or the lymphatic system to more distant locations. Yet, lymphovascular invasion is generally the first step of metastasis.
The host response to cancer therapy is defined as a physiological response of the non-malignant cells of the body to a specific cancer therapy. The response is therapy-specific, occurring independently of cancer type or stage.
Mammary secretory carcinoma (MSC), also termed secretory carcinoma of the breast, is a rare form of the breast cancers. MSC usually affects women but in a significant percentage of cases also occurs in men and children. Indeed, McDvitt and Stewart first described MSC in 1966 and termed it juvenile breast carcinoma because an increased number of cases were at that time diagnosed in juvenile females. MSC is the most common form of breast cancer in children, representing 80% of childhood breast cancers, although it accounts for less than 0.15% of all breast cancers.












