
An axon, or nerve fiber, is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction can be the cause of many inherited and acquired neurological disorders that affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.

Myelin is a lipid-rich material that surrounds nerve cell axons to insulate them and increase the rate at which electrical impulses pass along the axon. The myelinated axon can be likened to an electrical wire with insulating material (myelin) around it. However, unlike the plastic covering on an electrical wire, myelin does not form a single long sheath over the entire length of the axon. Rather, myelin ensheaths the axon segmentally: in general, each axon is encased in multiple long sheaths with short gaps between, called nodes of Ranvier. At the nodes of Ranvier, which are approximately one thousandth of a mm in length, the axon's membrane is bare of myelin.

Within a nervous system, a neuron, neurone, or nerve cell is an electrically excitable cell that fires electric signals called action potentials across a neural network. Neurons communicate with other cells via synapses, which are specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap.
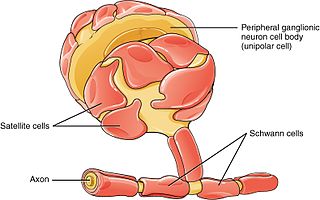
Schwann cells or neurolemmocytes are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle. The two types of Schwann cells are myelinating and nonmyelinating. Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath. The Schwann cell promoter is present in the downstream region of the human dystrophin gene that gives shortened transcript that are again synthesized in a tissue-specific manner.
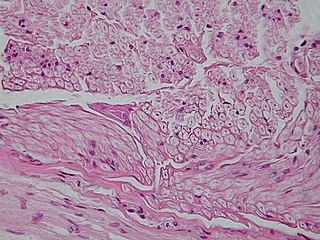
Nervous tissue, also called neural tissue, is the main tissue component of the nervous system. The nervous system regulates and controls body functions and activity. It consists of two parts: the central nervous system (CNS) comprising the brain and spinal cord, and the peripheral nervous system (PNS) comprising the branching peripheral nerves. It is composed of neurons, also known as nerve cells, which receive and transmit impulses, and neuroglia, also known as glial cells or glia, which assist the propagation of the nerve impulse as well as provide nutrients to the neurons.
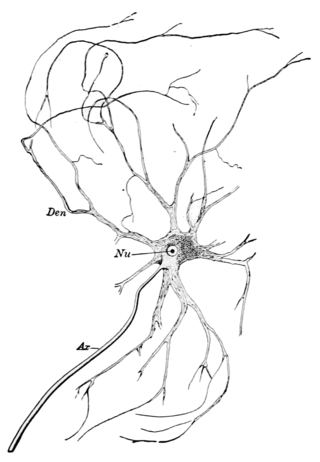
A motor nerve is a nerve that transmits motor signals from the central nervous system (CNS) to the muscles of the body. This is different from the motor neuron, which includes a cell body and branching of dendrites, while the nerve is made up of a bundle of axons. Motor nerves act as efferent nerves which carry information out from the CNS to muscles, as opposed to afferent nerves, which transfer signals from sensory receptors in the periphery to the CNS. Efferent nerves can also connect to glands or other organs/issues instead of muscles. In addition, there are nerves that serve as both sensory and motor nerves called mixed nerves.

Glia, also called glial cells(gliocytes) or neuroglia, are non-neuronal cells in the central nervous system (brain and spinal cord) and the peripheral nervous system that do not produce electrical impulses. The neuroglia make up more than one half the volume of neural tissue in our body. They maintain homeostasis, form myelin in the peripheral nervous system, and provide support and protection for neurons. In the central nervous system, glial cells include oligodendrocytes, astrocytes, ependymal cells and microglia, and in the peripheral nervous system they include Schwann cells and satellite cells.

Wallerian degeneration is an active process of degeneration that results when a nerve fiber is cut or crushed and the part of the axon distal to the injury degenerates. A related process of dying back or retrograde degeneration known as 'Wallerian-like degeneration' occurs in many neurodegenerative diseases, especially those where axonal transport is impaired such as ALS and Alzheimer's disease. Primary culture studies suggest that a failure to deliver sufficient quantities of the essential axonal protein NMNAT2 is a key initiating event.

Astrogliosis is an abnormal increase in the number of astrocytes due to the destruction of nearby neurons from central nervous system (CNS) trauma, infection, ischemia, stroke, autoimmune responses or neurodegenerative disease. In healthy neural tissue, astrocytes play critical roles in energy provision, regulation of blood flow, homeostasis of extracellular fluid, homeostasis of ions and transmitters, regulation of synapse function and synaptic remodeling. Astrogliosis changes the molecular expression and morphology of astrocytes, in response to infection for example, in severe cases causing glial scar formation that may inhibit axon regeneration.
Gliosis is a nonspecific reactive change of glial cells in response to damage to the central nervous system (CNS). In most cases, gliosis involves the proliferation or hypertrophy of several different types of glial cells, including astrocytes, microglia, and oligodendrocytes. In its most extreme form, the proliferation associated with gliosis leads to the formation of a glial scar.

Myelin-associated glycoprotein is a type 1 transmembrane protein glycoprotein localized in periaxonal Schwann cell and oligodendrocyte membranes, where it plays a role in glial-axonal interactions. MAG is a member of the SIGLEC family of proteins and is a functional ligand of the NOGO-66 receptor, NgR. MAG is believed to be involved in myelination during nerve regeneration in the PNS and is vital for the long-term survival of the myelinated axons following myelinogenesis. In the CNS MAG is one of three main myelin-associated inhibitors of axonal regeneration after injury, making it an important protein for future research on neurogenesis in the CNS.

Nerve injury is an injury to a nerve. There is no single classification system that can describe all the many variations of nerve injuries. In 1941, Seddon introduced a classification of nerve injuries based on three main types of nerve fiber injury and whether there is continuity of the nerve. Usually, however, nerve injuries are classified in five stages, based on the extent of damage to both the nerve and the surrounding connective tissue, since supporting glial cells may be involved.
A nerve guidance conduit is an artificial means of guiding axonal regrowth to facilitate nerve regeneration and is one of several clinical treatments for nerve injuries. When direct suturing of the two stumps of a severed nerve cannot be accomplished without tension, the standard clinical treatment for peripheral nerve injuries is autologous nerve grafting. Due to the limited availability of donor tissue and functional recovery in autologous nerve grafting, neural tissue engineering research has focused on the development of bioartificial nerve guidance conduits as an alternative treatment, especially for large defects. Similar techniques are also being explored for nerve repair in the spinal cord but nerve regeneration in the central nervous system poses a greater challenge because its axons do not regenerate appreciably in their native environment.
Nerve tissue is a biological molecule related to the function and maintenance of normal nervous tissue. An example would include, for example, the generation of myelin which insulates and protects nerves. These are typically calcium-binding proteins.

A glial scar formation (gliosis) is a reactive cellular process involving astrogliosis that occurs after injury to the central nervous system. As with scarring in other organs and tissues, the glial scar is the body's mechanism to protect and begin the healing process in the nervous system.
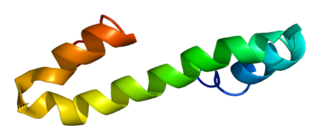
Reticulon 4, also known as Neurite outgrowth inhibitor or Nogo, is a protein that in humans is encoded by the RTN4 gene that has been identified as an inhibitor of neurite outgrowth specific to the central nervous system. During neural development Nogo is expressed mainly by neurons and provides an inhibitory signal for the migration and sprouting of CNS endothelial (tip) cells, thereby restricting blood vessel density.

Leucine rich repeat and Immunoglobin-like domain-containing protein 1 also known as LINGO-1 is a protein which is encoded by the LINGO1 gene in humans. It belongs to the family of leucine-rich repeat proteins which are known for playing key roles in the biology of the central nervous system. LINGO-1 is a functional component of the Nogo receptor also known as the reticulon 4 receptor.

Chondroitin sulfate proteoglycans (CSPGs) are proteoglycans consisting of a protein core and a chondroitin sulfate side chain. They are known to be structural components of a variety of human tissues, including cartilage, and also play key roles in neural development and glial scar formation. They are known to be involved in certain cell processes, such as cell adhesion, cell growth, receptor binding, cell migration, and interaction with other extracellular matrix constituents. They are also known to interact with laminin, fibronectin, tenascin, and collagen. CSPGs are generally secreted from cells.

Olfactory ensheathing cells (OECs), also known as olfactory ensheathing glia or olfactory ensheathing glial cells, are a type of macroglia found in the nervous system. They are also known as olfactory Schwann cells, because they ensheath the non-myelinated axons of olfactory neurons in a similar way to which Schwann cells ensheath non-myelinated peripheral neurons. They also share the property of assisting axonal regeneration.
Endogenous regeneration in the brain is the ability of cells to engage in the repair and regeneration process. While the brain has a limited capacity for regeneration, endogenous neural stem cells, as well as numerous pro-regenerative molecules, can participate in replacing and repairing damaged or diseased neurons and glial cells. Another benefit that can be achieved by using endogenous regeneration could be avoiding an immune response from the host.














