
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

Steelmaking is the process of producing steel from iron ore and/or scrap. In steelmaking, impurities such as nitrogen, silicon, phosphorus, sulfur and excess carbon are removed from the sourced iron, and alloying elements such as manganese, nickel, chromium, carbon and vanadium are added to produce different grades of steel.

Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.
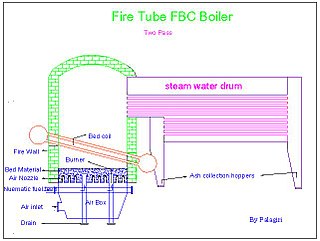
Fluidized bed combustion (FBC) is a combustion technology used to burn solid fuels.

A fossil fuel power station is a thermal power station which burns a fossil fuel, such as coal or natural gas, to produce electricity. Fossil fuel power stations have machinery to convert the heat energy of combustion into mechanical energy, which then operates an electrical generator. The prime mover may be a steam turbine, a gas turbine or, in small plants, a reciprocating gas engine. All plants use the energy extracted from the expansion of a hot gas, either steam or combustion gases. Although different energy conversion methods exist, all thermal power station conversion methods have their efficiency limited by the Carnot efficiency and therefore produce waste heat.

Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases, as from a fireplace, oven, furnace, boiler or steam generator. It often refers to the exhaust gas of combustion at power plants. Technology is available to remove pollutants from flue gas at power plants.

Coal pollution mitigation, sometimes labeled as clean coal, is a series of systems and technologies that seek to mitigate health and environmental impact of burning coal for energy. Burning coal releases harmful substances, including mercury, lead, sulfur dioxide (SO2), nitrogen oxides (NOx), and carbon dioxide (CO2), contributing to air pollution, acid rain, and greenhouse gas emissions. Methods include flue-gas desulfurization, selective catalytic reduction, electrostatic precipitators, and fly ash reduction focusing on reducing the emissions of these harmful substances. These measures aim to reduce coal's impact on human health and the environment.

A thermal power station is a type of power station in which heat energy is converted to electrical energy. In a steam-generating cycle heat is used to boil water in a large pressure vessel to produce high-pressure steam, which drives a steam turbine connected to an electrical generator. The low-pressure exhaust from the turbine enters a steam condenser where it is cooled to produce hot condensate which is recycled to the heating process to generate more high pressure steam. This is known as a Rankine cycle.

Carbon capture and storage (CCS) is a process in which a relatively pure stream of carbon dioxide (CO2) from industrial sources is separated, treated and transported to a long-term storage location. For example, the burning of fossil fuels or biomass results in a stream of CO2 that could be captured and stored by CCS. Usually the CO2 is captured from large point sources, such as a chemical plant or a bioenergy plant, and then stored in a suitable geological formation. The aim is to reduce greenhouse gas emissions and thus mitigate climate change. For example, CCS retrofits for existing power plants can be one of the ways to limit emissions from the electricity sector and meet the Paris Agreement goals.
An integrated gasification combined cycle (IGCC) is a technology using a high pressure gasifier to turn coal and other carbon based fuels into pressurized gas—synthesis gas (syngas). It can then remove impurities from the syngas prior to the electricity generation cycle. Some of these pollutants, such as sulfur, can be turned into re-usable byproducts through the Claus process. This results in lower emissions of sulfur dioxide, particulates, mercury, and in some cases carbon dioxide. With additional process equipment, a water-gas shift reaction can increase gasification efficiency and reduce carbon monoxide emissions by converting it to carbon dioxide. The resulting carbon dioxide from the shift reaction can be separated, compressed, and stored through sequestration. Excess heat from the primary combustion and syngas fired generation is then passed to a steam cycle, similar to a combined cycle gas turbine. This process results in improved thermodynamic efficiency, compared to conventional pulverized coal combustion.

A flue-gas stack, also known as a smoke stack, chimney stack or simply as a stack, is a type of chimney, a vertical pipe, channel or similar structure through, which combustion product gases called flue gases are exhausted to the outside air. Flue gases are produced when coal, oil, natural gas, wood or any other fuel is combusted in an industrial furnace, a power plant's steam-generating boiler, or other large combustion device. Flue gas is usually composed of carbon dioxide (CO2) and water vapor, as well as nitrogen and excess oxygen remaining from the intake combustion air. It also contains a small percentage of pollutants such as particulate matter, carbon monoxide, nitrogen oxides and sulfur oxides. The flue gas stacks are often quite tall, up to 420 metres (1,380 ft), to increase the stack effect and dispersion of pollutants.

A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage. They have also been researched for carbon capture and storage as a means of combating climate change.

Chemical looping combustion (CLC) is a technological process typically employing a dual fluidized bed system. CLC operated with an interconnected moving bed with a fluidized bed system, has also been employed as a technology process. In CLC, a metal oxide is employed as a bed material providing the oxygen for combustion in the fuel reactor. The reduced metal is then transferred to the second bed and re-oxidized before being reintroduced back to the fuel reactor completing the loop. Fig 1 shows a simplified diagram of the CLC process. Fig 2 shows an example of a dual fluidized bed circulating reactor system and a moving bed-fluidized bed circulating reactor system.
Carbon capture and storage (CCS) is a technology that can capture carbon dioxide CO2 emissions produced from fossil fuels in electricity, industrial processes which prevents CO2 from entering the atmosphere. Carbon capture and storage is also used to sequester CO2 filtered out of natural gas from certain natural gas fields. While typically the CO2 has no value after being stored, Enhanced Oil Recovery uses CO2 to increase yield from declining oil fields.

Bioenergy with carbon capture and storage (BECCS) is the process of extracting bioenergy from biomass and capturing and storing the carbon, thereby removing it from the atmosphere. BECCS can theoretically be a "negative emissions technology" (NET), although its deployment at the scale considered by many governments and industries can "also pose major economic, technological, and social feasibility challenges; threaten food security and human rights; and risk overstepping multiple planetary boundaries, with potentially irreversible consequences". The carbon in the biomass comes from the greenhouse gas carbon dioxide (CO2) which is extracted from the atmosphere by the biomass when it grows. Energy ("bioenergy") is extracted in useful forms (electricity, heat, biofuels, etc.) as the biomass is utilized through combustion, fermentation, pyrolysis or other conversion methods.
Post-combustion capture refers to the removal of carbon dioxide (CO2) from a power station flue gas prior to its compression, transportation and storage in suitable geological formations, as part of carbon capture and storage. A number of different techniques are applicable, almost all of which are adaptations of acid gas removal processes used in the chemical and petrochemical industries. Many of these techniques existed before World War II and, consequently, post-combustion capture is the most developed of the various carbon-capture methodologies.
Calcium looping (CaL), or the regenerative calcium cycle (RCC), is a second-generation carbon capture technology. It is the most developed form of carbonate looping, where a metal (M) is reversibly reacted between its carbonate form (MCO3) and its oxide form (MO) to separate carbon dioxide from other gases coming from either power generation or an industrial plant. In the calcium looping process, the two species are calcium carbonate (CaCO3) and calcium oxide (CaO). The captured carbon dioxide can then be transported to a storage site, used in enhanced oil recovery or used as a chemical feedstock. Calcium oxide is often referred to as the sorbent.
Lower-temperature fuel cell types such as the proton exchange membrane fuel cell, phosphoric acid fuel cell, and alkaline fuel cell require pure hydrogen as fuel, typically produced from external reforming of natural gas. However, fuels cells operating at high temperature such as the solid oxide fuel cell (SOFC) are not poisoned by carbon monoxide and carbon dioxide, and in fact can accept hydrogen, carbon monoxide, carbon dioxide, steam, and methane mixtures as fuel directly, because of their internal shift and reforming capabilities. This opens up the possibility of efficient fuel cell-based power cycles consuming solid fuels such as coal and biomass, the gasification of which results in syngas containing mostly hydrogen, carbon monoxide and methane which can be cleaned and fed directly to the SOFCs without the added cost and complexity of methane reforming, water gas shifting and hydrogen separation operations which would otherwise be needed to isolate pure hydrogen as fuel. A power cycle based on gasification of solid fuel and SOFCs is called an Integrated Gasification Fuel Cell (IGFC) cycle; the IGFC power plant is analogous to an integrated gasification combined cycle power plant, but with the gas turbine power generation unit replaced with a fuel cell power generation unit. By taking advantage of intrinsically high energy efficiency of SOFCs and process integration, exceptionally high power plant efficiencies are possible. Furthermore, SOFCs in the IGFC cycle can be operated so as to isolate a carbon dioxide-rich anodic exhaust stream, allowing efficient carbon capture to address greenhouse gas emissions concerns of coal-based power generation.
The Allam Cycle or Allam-Fetvedt Cycle is a process for converting carbonaceous fuels into thermal energy, while capturing the generated carbon dioxide and water. This zero emissions cycle was validated at a 50 MWth natural gas fed test facility in La Porte, Texas in May 2018. This industrial plant is owned and operated by NET Power LLC, a privately held technology licensing company. NET Power is owned by Constellation Energy Corporation, Occidental Petroleum Corporation (Oxy) Low Carbon Ventures, Baker Hughes Company and 8 Rivers Capital, the company holding the patents for the technology. The key inventors behind the process are English engineer Rodney John Allam, American engineer Jeremy Eron Fetvedt, American scientist Dr. Miles R Palmer, and American businessperson and innovator G. William Brown, Jr. The Allam-Fetvedt Cycle was recognized by MIT Technology Review on the 2018 list of 10 Breakthrough Technologies.













