
In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.

In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.
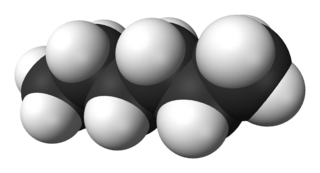
Hexane or n-hexane is an organic compound, a straight-chain alkane with six carbon atoms and the molecular formula C6H14.
1-Hexanol (IUPAC name hexan-1-ol) is an organic alcohol with a six-carbon chain and a condensed structural formula of CH3(CH2)5OH. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Two additional straight chain isomers of 1-hexanol, 2-hexanol and 3-hexanol, exist, both of which differing by the location of the hydroxyl group. Many isomeric alcohols have the formula C6H13OH. It is used in the perfume industry.

In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated.
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The chemical energy released in the formation of non-covalent interactions is typically on the order of 1–5 kcal/mol. Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects.
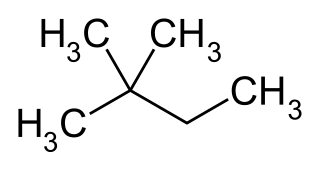
2,2-Dimethylbutane, trivially known as neohexane, is an organic compound with formula C6H14 or (H3C-)3-C-CH2-CH3. It is therefore an alkane, indeed the most compact and branched of the hexane isomers — the only one with a quaternary carbon and a butane (C4) backbone.

Vanadium oxytrichloride is the inorganic compound with the formula VOCl3. This yellow distillable liquid hydrolyzes readily in air. It is an oxidizing agent. It is used as a reagent in organic synthesis. Samples often appear red or orange owing to an impurity of vanadium tetrachloride.
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term diamine refers mostly to primary diamines, as those are the most reactive.
Candlenut oil or kukui nut oil is extracted from the nut of Aleurites moluccanus, the candlenut or kuku'i.
Pinacolyl alcohol is one of the isomeric hexanols and a secondary alcohol.
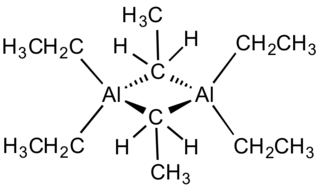
Triethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name the compound has the formula Al2(C2H5)6 (abbreviated as Al2Et6 or TEA). This colorless liquid is pyrophoric. It is an industrially important compound, closely related to trimethylaluminium.
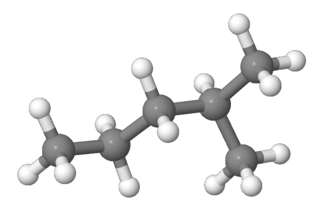
2-Methylpentane, trivially known as isohexane, is a branched-chain alkane with the molecular formula C6H14. It is a structural isomer of hexane composed of a methyl group bonded to the second carbon atom in a pentane chain. Using a quantitative structure-activity relationship (QSAR) prediction model, 2-Methylpentane has a research octane number (RON) of 75, motor octane number (MON) of 77, and cetane number (CN) of 29.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
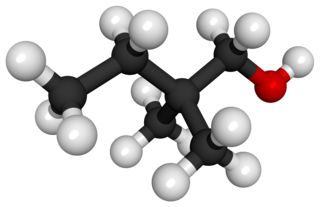
2,2-Dimethyl-1-butanol is an organic chemical compound; it is one of the isomeric hexanols. Its main use is as a solvent.
Base-promoted epoxide isomerization is the conversion of alkyl epoxides to ring-opened products through the action of strong base. Isomerizations of this type are most often used to synthesize allylic alcohols, although other products are possible.
Petroleum naphtha is an intermediate hydrocarbon liquid stream derived from the refining of crude oil with CAS-no 64742-48-9. It is most usually desulfurized and then catalytically reformed, which rearranges or restructures the hydrocarbon molecules in the naphtha as well as breaking some of the molecules into smaller molecules to produce a high-octane component of gasoline.

C70 fullerene is the fullerene molecule consisting of 70 carbon atoms. It is a cage-like fused-ring structure which resembles a rugby ball, made of 25 hexagons and 12 pentagons, with a carbon atom at the vertices of each polygon and a bond along each polygon edge. A related fullerene molecule, named buckminsterfullerene (or C60 fullerene) consists of 60 carbon atoms.
Iron(III) iodide is an inorganic compound with the chemical formula FeI3. It is a thermodynamically unstable compound that is difficult to prepare. Nevertheless, iron(III) iodide has been synthesised in small quantities in the absence of air and water.











