
Aciclovir, also known as acyclovir, is an antiviral medication. It is primarily used for the treatment of herpes simplex virus infections, chickenpox, and shingles. Other uses include prevention of cytomegalovirus infections following transplant and severe complications of Epstein–Barr virus infection. It can be taken by mouth, applied as a cream, or injected.

Valaciclovir, also spelled valacyclovir, is an antiviral medication used to treat outbreaks of herpes simplex or herpes zoster (shingles). It is also used to prevent cytomegalovirus following a kidney transplant in high risk cases. It is taken by mouth.

Gingivostomatitis is a combination of gingivitis and stomatitis, or an inflammation of the oral mucosa and gingiva. Herpetic gingivostomatitis is often the initial presentation during the first ("primary") herpes simplex infection. It is of greater severity than herpes labialis which is often the subsequent presentations. Primary herpetic gingivostomatitis is the most common viral infection of the mouth.

Famciclovir is a guanosine analogue antiviral drug used for the treatment of various herpesvirus infections, most commonly for herpes zoster (shingles). It is a prodrug form of penciclovir with improved oral bioavailability. Famciclovir is marketed under the trade name Famvir (Novartis).

Herpes simplex virus1 and 2, also known by their taxonomic names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are very common and contagious. They can be spread when an infected person begins shedding the virus.

Genital herpes is a herpes infection of the genitals caused by the herpes simplex virus (HSV). Most people either have no or mild symptoms and thus do not know they are infected. When symptoms do occur, they typically include small blisters that break open to form painful ulcers. Flu-like symptoms, such as fever, aching, or swollen lymph nodes, may also occur. Onset is typically around 4 days after exposure with symptoms lasting up to 4 weeks. Once infected further outbreaks may occur but are generally milder.
Herpes gladiatorum is one of the most infectious of herpes-caused diseases, and is transmissible by skin-to-skin contact. The disease was first described in the 1960s in the New England Journal of Medicine. It is caused by contagious infection with human herpes simplex virus type 1 (HSV-1), which more commonly causes oral herpes. Another strain, HSV-2 usually causes genital herpes, although the strains are very similar and either can cause herpes in any location.

Mollaret's meningitis is a recurrent or chronic inflammation of the protective membranes covering the brain and spinal cord, known collectively as the meninges. Since Mollaret's meningitis is a recurrent, benign (non-cancerous), aseptic meningitis, it is also referred to as benign recurrent lymphocytic meningitis. It was named for Pierre Mollaret, the French neurologist who first described it in 1944.
The central nervous system (CNS) controls most of the functions of the body and mind. It comprises the brain, spinal cord and the nerve fibers that branch off to all parts of the body. The CNS viral diseases are caused by viruses that attack the CNS. Existing and emerging viral CNS infections are major sources of human morbidity and mortality.

Herpes simplex, often known simply as herpes, is a viral infection caused by the herpes simplex virus. Herpes infections are categorized by the area of the body that is infected. The two major types of herpes are oral herpes and genital herpes, though other forms also exist.
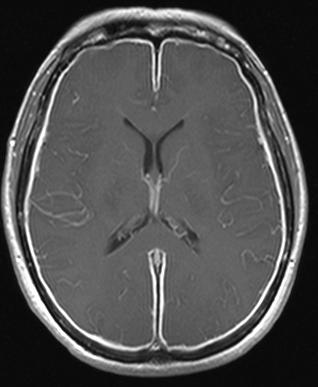
Herpes meningitis is inflammation of the meninges, the protective tissues surrounding the spinal cord and brain, due to infection from viruses of the Herpesviridae family - the most common amongst adults is HSV-2. Symptoms are self-limiting over 2 weeks with severe headache, nausea, vomiting, neck-stiffness, and photophobia. Herpes meningitis can cause Mollaret's meningitis, a form of recurrent meningitis. Lumbar puncture with cerebrospinal fluid results demonstrating aseptic meningitis pattern is necessary for diagnosis and polymerase chain reaction is used to detect viral presence. Although symptoms are self-limiting, treatment with antiviral medication may be recommended to prevent progression to Herpes Meningoencephalitis.

A cold sore is a type of herpes infection caused by the herpes simplex virus that affects primarily the lip. Symptoms typically include a burning pain followed by small blisters or sores. The first attack may also be accompanied by fever, sore throat, and enlarged lymph nodes. The rash usually heals within ten days, but the virus remains dormant in the trigeminal ganglion. The virus may periodically reactivate to create another outbreak of sores in the mouth or lip.
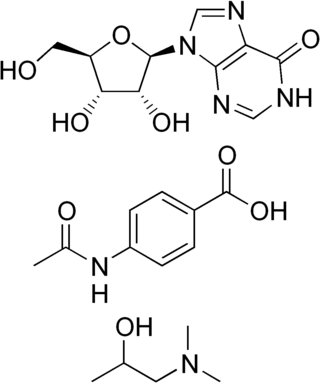
Inosine pranobex is an antiviral drug that is a combination of inosine and dimepranol acedoben in a ratio of 1 to 3. It is used primarily in European countries, especially as a treatment for acute viral infections, such as the common cold.
Herpes simplex research includes all medical research that attempts to prevent, treat, or cure herpes, as well as fundamental research about the nature of herpes. Examples of particular herpes research include drug development, vaccines and genome editing. HSV-1 and HSV-2 are commonly thought of as oral and genital herpes respectively, but other members in the herpes family include chickenpox (varicella/zoster), cytomegalovirus, and Epstein-Barr virus. There are many more virus members that infect animals other than humans, some of which cause disease in companion animals or have economic impacts in the agriculture industry.

Herpetic simplex keratitis is a form of keratitis caused by recurrent herpes simplex virus (HSV) infection in the cornea.

Talimogene laherparepvec, sold under the brand name Imlygic, is a biopharmaceutical medication used to treat melanoma that cannot be operated on; it is injected directly into a subset of lesions which generates a systemic immune response against the recipient's cancer. The final four year analysis from the pivotal phase 3 study upon which TVEC was approved by the FDA showed a 31.5% response rate with a 16.9% complete response (CR) rate. There was also a substantial and statistically significant survival benefit in patients with earlier metastatic disease and in patients who hadn't received prior systemic treatment for melanoma. The earlier stage group had a reduction in the risk of death of approximately 50% with one in four patients appearing to have met, or be close to be reaching, the medical definition of cure. Real world use of talimogene laherparepvec have shown response rates of up to 88.5% with CR rates of up to 61.5%.
TK is an experimental cell therapy which may be used to treat high-risk leukemia. It is currently undergoing a Phase III clinical trial to determine efficacy and clinical usefulness.

Pritelivir is a direct-acting antiviral drug in development for the treatment of herpes simplex virus infections (HSV). This is particularly important in immune compromised patients. Pritelivir is currently in Phase III clinical development by the German biopharmaceutical company AiCuris Anti-infective Cures AG.

Brincidofovir, sold under the brand name Tembexa, is an antiviral drug used to treat smallpox. Brincidofovir is a prodrug of cidofovir. Conjugated to a lipid, the compound is designed to release cidofovir intracellularly, allowing for higher intracellular and lower plasma concentrations of cidofovir, effectively increasing its activity against dsDNA viruses, as well as oral bioavailability.











