
Hepatitis is inflammation of the liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), poor appetite, vomiting, tiredness, abdominal pain, and diarrhea. Hepatitis is acute if it resolves within six months, and chronic if it lasts longer than six months. Acute hepatitis can resolve on its own, progress to chronic hepatitis, or (rarely) result in acute liver failure. Chronic hepatitis may progress to scarring of the liver (cirrhosis), liver failure, and liver cancer.
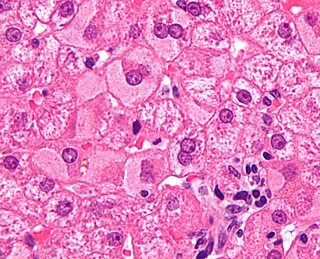
Viral hepatitis is liver inflammation due to a viral infection. It may present in acute form as a recent infection with relatively rapid onset, or in chronic form, typically progressing from a long-lasting asymptomatic condition up to a decompensated hepatic disease and hepatocellular carcinoma (HCC).
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.

Lamivudine, commonly called 3TC, is an antiretroviral medication used to prevent and treat HIV/AIDS. It is also used to treat chronic hepatitis B when other options are not possible. It is effective against both HIV-1 and HIV-2. It is typically used in combination with other antiretrovirals such as zidovudine, dolutegravir, and abacavir. Lamivudine may be included as part of post-exposure prevention in those who have been potentially exposed to HIV. Lamivudine is taken by mouth as a liquid or tablet.

Emtricitabine, with trade name Emtriva, is a nucleoside reverse-transcriptase inhibitor (NRTI) for the prevention and treatment of HIV infection in adults and children. In 2019, it was the 494th most commonly prescribed medication in the United States, with more than 3 thousand prescriptions.
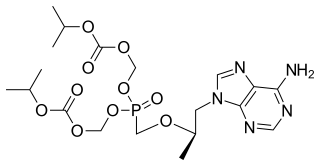
Tenofovir disoproxil, sold under the trade name Viread among others, is a medication used to treat chronic hepatitis B and to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention of HIV/AIDS among those at high risk before exposure, and after a needlestick injury or other potential exposure. It is sold both by itself and together in combinations such as emtricitabine/tenofovir, efavirenz/emtricitabine/tenofovir, and elvitegravir/cobicistat/emtricitabine/tenofovir. It does not cure HIV/AIDS or hepatitis B. It is available by mouth as a tablet or powder.

Adefovir is a prescription medicine used to treat (chronic) infections with hepatitis B virus. A prodrug form of adefovir was previously called bis-POM PMEA, with trade names Preveon and Hepsera. It is an orally administered nucleotide analog reverse-transcriptase inhibitor (ntRTI). It can be formulated as the pivoxil prodrug adefovir dipivoxil.

Nucleoside analogues are structural analogues of a nucleoside, which normally contain a nucleobase and a sugar. Nucleotide analogues are analogues of a nucleotide, which normally has one to three phosphates linked to a nucleoside. Both types of compounds can deviate from what they mimick in a number of ways, as changes can be made to any of the constituent parts. They are related to nucleic acid analogues.

Lamivudine/zidovudine, sold under the brand name Combivir among others, is a fixed-dose combination antiretroviral medication used to treat HIV/AIDS. It contains two antiretroviral medications, lamivudine and zidovudine. It is used together with other antiretrovirals. It is taken by mouth twice a day.
Hepatitis B is endemic in China. Of the 350 million individuals worldwide infected with the hepatitis B virus (HBV), one-third reside in China. As of 2006 China has immunized 11.1 million children in its poorest provinces as part of several programs initiated by the Chinese government and as part of the Global Alliance for Vaccines and Immunization (GAVI). However, the effects of these programs have yet to reach levels of immunization that would limit the spread of hepatitis B effectively.

Nitazoxanide, sold under the brand name Alinia among others, is a broad-spectrum antiparasitic and broad-spectrum antiviral medication that is used in medicine for the treatment of various helminthic, protozoal, and viral infections. It is indicated for the treatment of infection by Cryptosporidium parvum and Giardia lamblia in immunocompetent individuals and has been repurposed for the treatment of influenza. Nitazoxanide has also been shown to have in vitro antiparasitic activity and clinical treatment efficacy for infections caused by other protozoa and helminths; evidence as of 2014 suggested that it possesses efficacy in treating a number of viral infections as well.

Telbivudine is an antiviral drug used in the treatment of hepatitis B infection. It is marketed by Swiss pharmaceutical company Novartis under the trade names Sebivo and Tyzeka. Clinical trials have shown it to be significantly more effective than lamivudine or adefovir, and less likely to cause resistance. However, HBV signature resistance mutation M204I or L180M+M204V have been associated with Telbivudine resistance.

Elvucitabine is an experimental nucleoside reverse transcriptase inhibitor (NRTI), developed by Achillion Pharmaceuticals, Inc. for the treatment of HIV infection.

Hepatitis B is an infectious disease caused by the Hepatitis B virus (HBV) that affects the liver; it is a type of viral hepatitis. It can cause both acute and chronic infection.

Hepatitis B virus (HBV) is a partially double-stranded DNA virus, a species of the genus Orthohepadnavirus and a member of the Hepadnaviridae family of viruses. This virus causes the disease hepatitis B.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.

A precore mutant is a variety of hepatitis B virus that does not produce hepatitis B virus e antigen (HBeAg). These mutants are important because infections caused by these viruses are difficult to treat, and can cause infections of prolonged duration and with a higher risk of liver cirrhosis. The mutations are changes in DNA bases from guanine to adenine at base position 1896 (G1896A), and from cytosine to thymine at position 1858 (C1858T) in the precore region of the viral genome.

Carbocyclic nucleosides are nucleoside analogues in which a methylene group has replaced the oxygen atom of the furanose ring. These analogues have the nucleobase attached at a simple alkyl carbon rather than being part of a hemiaminal ether linkage. As a result, they have increased chemical stability. They also have increased metabolic stability because they are unaffected by phosphorylases and hydrolases that cleave the glycosidic bond between the nucleobase and furanose ring of nucleosides. They retain many of the biological properties of the original nucleosides with respect to recognition by various enzymes and receptors.

Non-structural protein 5B (NS5B) inhibitors are a class of direct-acting antivirals widely used in the treatment of chronic hepatitis C. Depending on site of action and chemical composition, NS5B inhibitors may be categorized into three classes—nucleoside active site inhibitors (NIs), non-nucleoside allosteric inhibitors, and pyrophosphate analogues. Subsequently, all three classes are then subclassified. All inhibit RNA synthesis by NS5B but at different stages/sites resulting in inability of viral RNA replication. Expression of direct-acting NS5B inhibitors does not take place in cells that are not infected by hepatitis C virus, which seems to be beneficial for this class of drugs.

Lobucavir is an antiviral drug that shows broad-spectrum activity against herpesviruses, hepatitis B and other hepadnaviruses, HIV/AIDS and cytomegalovirus. It initially demonstrated positive results in human clinical trials against hepatitis B with minimal adverse effects but was discontinued from further development following the discovery of increased risk of cancer associated with long-term use in mice. Although this carcinogenic risk is present in other antiviral drugs, such as zidovudine and ganciclovir that have been approved for clinical use, development was halted by Bristol-Myers Squibb, its manufacturer.


















