Varicella zoster virus (VZV), also known as human herpesvirus 3 or Human alphaherpesvirus 3 (taxonomically), is one of nine known herpes viruses that can infect humans. It causes chickenpox (varicella) commonly affecting children and young adults, and shingles in adults but rarely in children. As a late complication of VZV infection, Ramsay Hunt syndrome type 2 may develop in rare cases. VZV infections are species-specific to humans. The virus can survive in external environments for a few hours.

Shingles, also known as herpes zoster, is a viral disease characterized by a painful skin rash with blisters in a localized area. Typically the rash occurs in a single, wide mark either on the left or right side of the body or face. Two to four days before the rash occurs there may be tingling or local pain in the area. Other common symptoms are fever, headache, and tiredness. The rash usually heals within two to four weeks; however, some people develop ongoing nerve pain which can last for months or years, a condition called postherpetic neuralgia (PHN). In those with poor immune function the rash may occur widely. If the rash involves the eye, vision loss may occur.

Keratitis is a condition in which the eye's cornea, the clear dome on the front surface of the eye, becomes inflamed. The condition is often marked by moderate to intense pain and usually involves any of the following symptoms: pain, impaired eyesight, photophobia, red eye and a 'gritty' sensation. Diagnosis of infectious keratitis is usually made clinically based on the signs and symptoms as well as eye examination, but corneal scrapings may be obtained and evaluated using microbiological culture or other testing to identify the causative pathogen.

Aciclovir, also known as acyclovir, is an antiviral medication. It is primarily used for the treatment of herpes simplex virus infections, chickenpox, and shingles. Other uses include prevention of cytomegalovirus infections following transplant and severe complications of Epstein–Barr virus infection. It can be taken by mouth, applied as a cream, or injected.

Valaciclovir, also spelled valacyclovir, is an antiviral medication used to treat outbreaks of herpes simplex or herpes zoster (shingles). It is also used to prevent cytomegalovirus following a kidney transplant in high risk cases. It is taken by mouth.

Thymidine kinase is an enzyme, a phosphotransferase : 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, EC 2.7.1.21. It can be found in most living cells. It is present in two forms in mammalian cells, TK1 and TK2. Certain viruses also have genetic information for expression of viral thymidine kinases. Thymidine kinase catalyzes the reaction:

Vidarabine or 9-β-D-arabinofuranosyladenine (ara-A) is an antiviral drug which is active against herpes simplex and varicella zoster viruses.

Nucleoside analogues are structural analogues of a nucleoside, which normally contain a nucleobase and a sugar. Nucleotide analogues are analogues of a nucleotide, which normally has one to three phosphates linked to a nucleoside. Both types of compounds can deviate from what they mimick in a number of ways, as changes can be made to any of the constituent parts. They are related to nucleic acid analogues.

Herpes simplex virus1 and 2, also known by their taxonomic names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are very common and contagious. They can be spread when an infected person begins shedding the virus.

Idoxuridine is an anti-herpesvirus antiviral drug.

Trifluridine is an anti-herpesvirus antiviral drug, used primarily on the eye. It was sold under the trade name Viroptic by Glaxo Wellcome, now merged into GlaxoSmithKline. The brand is now owned by Monarch Pharmaceuticals, which is wholly owned by King Pharmaceuticals.

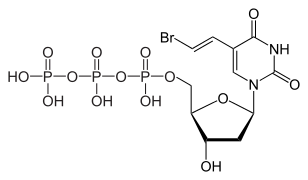
Sorivudine (INN), is a nucleoside analogue antiviral drug, marketed under trade names such as Usevir and Brovavir (BMS). It is used for the treatment of varicella zoster virus infections.

Chickenpox, also known as varicella, is a highly contagious, vaccine-preventable disease caused by the initial infection with varicella zoster virus (VZV), a member of the herpesvirus family. The disease results in a characteristic skin rash that forms small, itchy blisters, which eventually scab over. It usually starts on the chest, back, and face. It then spreads to the rest of the body. The rash and other symptoms, such as fever, tiredness, and headaches, usually last five to seven days. Complications may occasionally include pneumonia, inflammation of the brain, and bacterial skin infections. The disease is usually more severe in adults than in children.

Thymidine kinase from herpesvirus is a sub-family of thymidine kinases that catalyses the transfer of phospho group of ATP to thymidine to generate thymidine monophosphate, which serves as a substrate during viral DNA replication.

Herpes simplex, often known simply as herpes, is a viral infection caused by the herpes simplex virus. Herpes infections are categorized by the area of the body that is infected. The two major types of herpes are oral herpes and genital herpes, though other forms also exist.
In medicine, varicella zoster virus globulin, VZV antibodies, zoster immunoglobulin (ZIG), varicella zoster immune globulin, is an immune system medication that is used mostly for immunosuppressed patients who have been or may be exposed to the varicella zoster virus (VZV).

Herpetic simplex keratitis is a form of keratitis caused by recurrent herpes simplex virus (HSV) infection in the cornea.

Pritelivir is a direct-acting antiviral drug in development for the treatment of herpes simplex virus infections (HSV). This is particularly important in immune compromised patients. Pritelivir is currently in Phase III clinical development by the German biopharmaceutical company AiCuris Anti-infective Cures AG.

Carbocyclic nucleosides are nucleoside analogues in which a methylene group has replaced the oxygen atom of the furanose ring. These analogues have the nucleobase attached at a simple alkyl carbon rather than being part of a hemiaminal ether linkage. As a result, they have increased chemical stability. They also have increased metabolic stability because they are unaffected by phosphorylases and hydrolases that cleave the glycosidic bond between the nucleobase and furanose ring of nucleosides. They retain many of the biological properties of the original nucleosides with respect to recognition by various enzymes and receptors.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.