
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions.

The Philadelphia chromosome or Philadelphia translocation (Ph) is a specific genetic abnormality in chromosome 22 of leukemia cancer cells. This chromosome is defective and unusually short because of reciprocal translocation, t(9;22)(q34;q11), of genetic material between chromosome 9 and chromosome 22, and contains a fusion gene called BCR-ABL1. This gene is the ABL1 gene of chromosome 9 juxtaposed onto the breakpoint cluster region BCR gene of chromosome 22, coding for a hybrid protein: a tyrosine kinase signaling protein that is "always on", causing the cell to divide uncontrollably by interrupting the stability of the genome and impairing various signaling pathways governing the cell cycle.

Chronic myelogenous leukemia (CML), also known as chronic myeloid leukemia, is a cancer of the white blood cells. It is a form of leukemia characterized by the increased and unregulated growth of myeloid cells in the bone marrow and the accumulation of these cells in the blood. CML is a clonal bone marrow stem cell disorder in which a proliferation of mature granulocytes and their precursors is found. It is a type of myeloproliferative neoplasm associated with a characteristic chromosomal translocation called the Philadelphia chromosome.

Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral targeted therapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple receptor tyrosine kinases such as CSF1R, ABL, c-KIT, FLT3, and PDGFR-β. Specifically, it is used for chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL) that are Philadelphia chromosome–positive (Ph+), certain types of gastrointestinal stromal tumors (GIST), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), systemic mastocytosis, and myelodysplastic syndrome.

Erlotinib, sold under the brand name Tarceva among others, is a medication used to treat non-small cell lung cancer (NSCLC) and pancreatic cancer. Specifically it is used for NSCLC with mutations in the epidermal growth factor receptor (EGFR) — either an exon 19 deletion (del19) or exon 21 (L858R) substitution mutation — which has spread to other parts of the body. It is taken by mouth.

Nilotinib, sold under the brand name Tasigna marketed worldwide by Novartis, is a medication used to treat chronic myelogenous leukemia (CML) which has the Philadelphia chromosome. It may be used both in initial cases of chronic phase CML as well as in accelerated and chronic phase CML that has not responded to imatinib. It is taken by mouth.

Dasatinib, sold under the brand name Sprycel among others, is a targeted therapy medication used to treat certain cases of chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL). Specifically it is used to treat cases that are Philadelphia chromosome-positive (Ph+). It is taken by mouth.
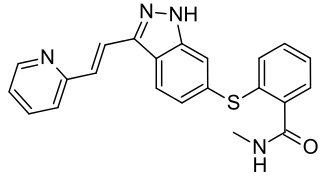
Axitinib, sold under the brand name Inlyta, is a small molecule tyrosine kinase inhibitor developed by Pfizer. It has been shown to significantly inhibit growth of breast cancer in animal (xenograft) models and has shown partial responses in clinical trials with renal cell carcinoma (RCC) and several other tumour types.

Proto-oncogene tyrosine-protein kinase ROS is an enzyme that in humans is encoded by the ROS1 gene.
Spectrum Pharmaceuticals is an American biopharmaceutical company. The company is located in Boston, MA.

Crizotinib, sold under the brand name Xalkori among others, is an anti-cancer medication used for the treatment of non-small cell lung carcinoma (NSCLC). It acts as an ALK and ROS1 inhibitor.

ALK inhibitors are anti-cancer drugs that act on tumours with variations of anaplastic lymphoma kinase (ALK) such as an EML4-ALK translocation. They fall under the category of tyrosine kinase inhibitors, which work by inhibiting proteins involved in the abnormal growth of tumour cells. All the current approved ALK inhibitors function by binding to the ATP pocket of the abnormal ALK protein, blocking its access to energy and deactivating it. A majority of ALK-rearranged NSCLC harbour the EML4-ALK fusion, although as of 2020, over 92 fusion partners have been discovered in ALK+ NSCLC. For each fusion partner, there can be several fusion variants depending on the position the two genes were fused at, and this may have implications on the response of the tumour and prognosis of the patient.
Bcr-Abl tyrosine-kinase inhibitors (TKI) are the first-line therapy for most patients with chronic myelogenous leukemia (CML). More than 90% of CML cases are caused by a chromosomal abnormality that results in the formation of a so-called Philadelphia chromosome. This abnormality was discovered by Peter Nowell in 1960 and is a consequence of fusion between the Abelson (Abl) tyrosine kinase gene at chromosome 9 and the break point cluster (Bcr) gene at chromosome 22, resulting in a chimeric oncogene (Bcr-Abl) and a constitutively active Bcr-Abl tyrosine kinase that has been implicated in the pathogenesis of CML. Compounds have been developed to selectively inhibit the tyrosine kinase.
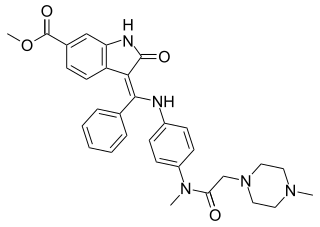
Nintedanib, sold under the brand names Ofev and Vargatef, is an oral medication used for the treatment of idiopathic pulmonary fibrosis and along with other medications for some types of non-small-cell lung cancer.

Ponatinib, sold under the brand name Iclusig, is a medication developed by ARIAD Pharmaceuticals for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). It is a multi-targeted tyrosine-kinase inhibitor. Some forms of CML, those that have the T315I mutation, are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors.

Brigatinib, sold under the brand name Alunbrig among others, is a small-molecule targeted cancer therapy being developed by Ariad Pharmaceuticals, Inc. Brigatinib acts as both an anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) inhibitor.
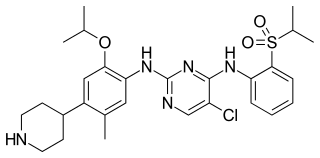
Ceritinib is a prescription-only drug used for the treatment of non-small cell lung cancer (NSCLC). It was developed by Novartis and received FDA approval for use in April 2014..Ceritinib is also sold under the brand name Spexib in few countries by Novartis.

Osimertinib, sold under the brand name Tagrisso, is a medication used to treat non-small-cell lung carcinomas with specific mutations. It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor.

Alectinib (INN,), sold under the brand name Alecensa, is a medication that is used to treat non-small-cell lung cancer (NSCLC). It blocks the activity of anaplastic lymphoma kinase (ALK). It is taken by mouth. It was developed by Chugai Pharmaceutical Co. Japan, which is part of the Hoffmann-La Roche group.
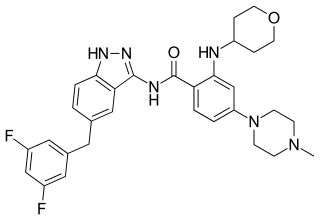
Entrectinib, sold under the brand name Rozlytrek, is an anti-cancer medication used to treat ROS1-positive non-small cell lung cancer and NTRK fusion-positive solid tumors. It is a selective tyrosine kinase inhibitor (TKI), of the tropomyosin receptor kinases (TRK) A, B and C, C-ros oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK).

















