Drugs used in diabetes treat diabetes mellitus by decreasing the glucose level in the blood. With the exception of insulin, most GLP receptor agonists, and pramlintide, all are administered orally and are thus also called oral hypoglycemic agents or oral antihyperglycemic agents. There are different classes of hypoglycemic drugs, and their selection depends on the nature of diabetes, age, and situation of the person, as well as other factors.

AstraZeneca plc is an Anglo-Swedish multinational pharmaceutical and biotechnology company with its headquarters at the Cambridge Biomedical Campus in Cambridge, England. It has a portfolio of products for major diseases in areas including oncology, cardiovascular, gastrointestinal, infection, neuroscience, respiratory, and inflammation. It has been involved in developing the Oxford–AstraZeneca COVID-19 vaccine.

The Bristol-Myers Squibb Company, doing business as Bristol Myers Squibb (BMS), is an American multinational pharmaceutical company. Headquartered in Princeton, New Jersey, BMS is one of the world's largest pharmaceutical companies and consistently ranks on the Fortune 500 list of the largest U.S. corporations. For fiscal 2022, it had a total revenue of $46.2 billion.
Sanofi S.A. is a French multinational pharmaceutical and healthcare company headquartered in Paris, France. Originally, the corporation was established in 1973 and merged with Synthélabo in 1999 to form Sanofi-Synthélabo. In 2004, Sanofi-Synthélabo merged with Aventis and renamed to Sanofi-Aventis, which were each the product of several previous mergers. It changed its name back to Sanofi in May 2011. The company is a component of the Euro Stoxx 50 stock market index.

Stavudine (d4T), sold under the brand name Zerit among others, is an antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. However, it is not a first-line treatment. It is given by mouth.

Amylin, or islet amyloid polypeptide (IAPP), is a 37-residue peptide hormone. It is co-secreted with insulin from the pancreatic β-cells in the ratio of approximately 100:1 (insulin:amylin). Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.

Biocon Limited is an Indian biopharmaceutical company based in Bangalore. It was founded by Kiran Mazumdar-Shaw in 1978. The company manufactures generic active pharmaceutical ingredients (APIs) that are sold in approximately 120 countries, including the United States and Europe. It also manufactures novel biologics as well as biosimilar insulins and antibodies, which are sold in India as branded formulations. Biocon's biosimilar products are also sold in both bulk and formulation forms in several emerging markets.

Pramlintide is an injectable amylin analogue drug for diabetes, developed by Amylin Pharmaceuticals. Pramlintide is sold as an acetate salt.

Celgene Corporation is a pharmaceutical company that makes cancer and immunology drugs. Its major product is Revlimid (lenalidomide), which is used in the treatment of multiple myeloma, and also in certain anemias. The company is incorporated in Delaware, headquartered in Summit, New Jersey, and a subsidiary of Bristol Myers Squibb (BMS).

Exenatide, sold under the brand name Byetta and Bydureon among others, is a medication used to treat diabetes mellitus type 2. It is used together with diet, exercise, and potentially other antidiabetic medication. It is a treatment option after metformin and sulfonylureas. It is given by injection under the skin twice daily or once weekly.

Saxagliptin, sold under the brand name Onglyza, is an oral hypoglycemic of the dipeptidyl peptidase-4 (DPP-4) inhibitor class. Early development was solely by Bristol-Myers Squibb; in 2007 AstraZeneca joined with Bristol-Myers Squibb to co-develop the final compound and collaborate on the marketing of the drug.
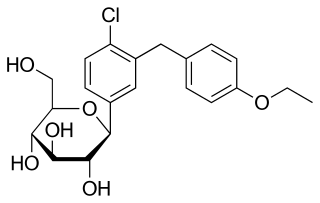
Dapagliflozin, sold under the brand names Farxiga (US) and Forxiga (EU) among others, is a medication used to treat type 2 diabetes. It is also used to treat adults with heart failure and chronic kidney disease.

Insulin lispro, sold under the brand name Humalog among others, is a modified type of medical insulin used to treat type 1 and type 2 diabetes. It is used by injection under the skin or within an insulin pump. Onset of effects typically occurs within 30 minutes and lasts about 5 hours. Often a longer-acting insulin like insulin NPH is also needed.

Muraglitazar is a dual peroxisome proliferator-activated receptor agonist with affinity to PPARα and PPARγ.
Glucagon-like peptide-1 (GLP-1) receptor agonists, also known as GLP-1 analogs, GLP-1DAs or incretin mimetics, are a class of drugs that reduce blood sugar and energy intake by activating the GLP-1 receptor. They mimic the actions of the endogenous incretin hormone GLP-1 that is released by the gut after eating.
Acquired generalized lipodystrophy is a rare skin condition that appears during childhood or adolescence, characterized by fat loss affecting large areas of the body, particularly the face, arms, and legs. There are 4 types of lipodystrophy based on its onset and areas affected: acquired or inherited, and generalized or partial. Both acquired or inherited lipodystrophy present as loss of adipose tissues, in the absence of nutritional deprivation. The near-total loss of subcutaneous adipose tissue is termed generalized lipodystrophy while the selective loss of adipose tissues is denoted as partial lipodystrophy. Thus, as the name suggests, AGL is a near-total deficiency of adipose tissues in the body that is developed later in life. It is an extremely rare disease that only about 100 cases are reported worldwide. There are three main etiologies of AGL suspected: autoimmune, panniculitis-associated, or idiopathic. After its onset, the disease progresses over a few days, weeks, months, or even in years. Clinical presentations of AGL are similar to other lipodystrophies, including metabolic complications and hypoleptinemia. Treatments are also similar and mainly supportive for symptomatic alleviation. Although HIV- or drug-induced lipodystrophy are a type of acquired lipodystrophy, its origin is very specific and distinct hence is usually not discussed with AGL.

Insulin degludec (INN/USAN) is an ultralong-acting basal insulin analogue that was developed by Novo Nordisk under the brand name Tresiba. It is administered via subcutaneous injection once daily to help control the blood sugar level of those with diabetes. It has a duration of action that lasts up to 42 hours, making it a once-daily basal insulin, that is one that provides a base insulin level, as opposed to the fast- and short-acting bolus insulins.
Metreleptin, sold under the brand name Myalept among others, is a synthetic analog of the hormone leptin used to treat various forms of dyslipidemia. It has been approved in Japan for metabolic disorders including lipodystrophy and in the United States as replacement therapy to treat the complications of leptin deficiency, in addition to diet, in patients with congenital generalized or acquired generalized lipodystrophy.
Dapagliflozin/saxagliptin/metformin, sold under the brand name Qternmet XR among others, is a fixed-dose combination anti-diabetic medication used as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. It is a combination of dapagliflozin, saxagliptin, and metformin. It is taken by mouth. The drug is marketed by AstraZeneca.













