
Biogen Inc. is an American multinational biotechnology company based in Cambridge, Massachusetts, United States specializing in the discovery, development, and delivery of therapies for the treatment of neurological diseases to patients worldwide. Biogen operates in Argentina, Brazil, Canada, China, France, Germany, Hungary, India, Italy, Japan, Mexico, Netherlands, Poland, Sweden, and Switzerland.

l-DOPA, also known as levodopa and l-3,4-dihydroxyphenylalanine, is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine. l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS. In some plant families, l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains. l-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.

Amantadine, sold under the brand name Gocovri among others, is a medication used to treat dyskinesia associated with parkinsonism and influenza caused by type A influenzavirus, though its use for the latter is no longer recommended because of widespread drug resistance. It acts as a nicotinic antagonist, dopamine agonist, and noncompetitive NMDA antagonist. The antiviral mechanism of action is antagonism of the influenzavirus A M2 proton channel, which prevents endosomal escape.
Antisense therapy is a form of treatment that uses antisense oligonucleotides (ASOs) to target messenger RNA (mRNA). ASOs are capable of altering mRNA expression through a variety of mechanisms, including ribonuclease H mediated decay of the pre-mRNA, direct steric blockage, and exon content modulation through splicing site binding on pre-mRNA. Several ASOs have been approved in the United States, the European Union, and elsewhere.

4-Aminopyridine (4-AP, fampridine, dalfampridine) is an organic compound with the chemical formula C5H4N–NH2. The molecule is one of the three isomeric amines of pyridine. It is used as a research tool in characterizing subtypes of the potassium channel. It has also been used as a drug, to manage some of the symptoms of multiple sclerosis, and is indicated for symptomatic improvement of walking in adults with several variations of the disease. It was undergoing Phase III clinical trials as of 2008, and the U.S. Food and Drug Administration (FDA) approved the compound on January 22, 2010. Fampridine is also marketed as Ampyra (pronounced "am-PEER-ah," according to the maker's website) in the United States by Acorda Therapeutics and as Fampyra in the European Union, Canada, and Australia. In Canada, the medication has been approved for use by Health Canada since February 10, 2012.

Entacapone, sold under the brand name Comtan among others, is a medication commonly used in combination with other medications for the treatment of Parkinson's disease. Entacapone together with levodopa and carbidopa allows levodopa to have a longer effect in the brain and reduces Parkinson's disease signs and symptoms for a greater length of time than levodopa and carbidopa therapy alone.
Inhalable insulin is a powdered form of insulin, delivered with an inhaler into the lungs where it is absorbed. In general inhaled insulins have been more rapidly absorbed than subcutaneous injected insulin, with faster peak concentration in serum and more rapid metabolism.
Cephalon, Inc. was an American biopharmaceutical company co-founded in 1987 by pharmacologist Frank Baldino, Jr., neuroscientist Michael Lewis, and organic chemist James C. Kauer—all three former scientists with the DuPont Company. Baldino served as Cephalon's chairman and chief executive officer, until his death in December 2010. The company's name comes from the adjective "cephalic" meaning "related to the head or brain", as it was established primarily to pursue treatments for neurodegenerative diseases.

Biotie Therapies was a Finnish biotechnology and pharmaceutics company that was acquired by Acorda Therapeutics in January 2016. The company's research and development was focused on drugs for neurodegenerative and psychiatric disorders like Parkinson's disease, Alzheimer's disease and other cognitive disorders, alcohol and drug dependence and post traumatic stress disorder, and inflammatory and fibrotic liver disease. The company's headquarters is in Turku, Western Finland, and it is listed on NASDAQ OMX Helsinki.

Droxidopa is a synthetic amino acid precursor which acts as a prodrug to the neurotransmitter norepinephrine (noradrenaline). Unlike norepinephrine, droxidopa is capable of crossing the protective blood–brain barrier (BBB).

Remacemide is a drug which acts as a low-affinity NMDA antagonist with sodium channel blocking properties. It has been studied for the treatment of acute ischemic stroke, epilepsy, Huntington's disease, and Parkinson's disease.

Safinamide is a drug used as an add-on treatment for Parkinson's disease with "off" episodes; it has multiple modes of action, including the inhibition of monoamine oxidase B.

Pimavanserin, sold under the brand name Nuplazid, is an atypical antipsychotic which is approved for the treatment of Parkinson's disease psychosis and is also being studied for the treatment of Alzheimer's disease psychosis, schizophrenia, agitation, and major depressive disorder. Unlike other antipsychotics, pimavanserin is not a dopamine receptor antagonist.
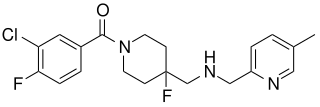
Befiradol is an experimental drug being studied for the treatment of levodopa-induced dyskinesia. It is a potent and selective 5-HT1A receptor full agonist.
Acadia Pharmaceuticals Inc. is a biopharmaceutical company headquartered in Sorrento Valley, San Diego, California.
Teprotumumab, sold under the brand name Tepezza, is a medication used to treat adults with thyroid eye disease, a rare condition where the muscles and fatty tissues behind the eye become inflamed, causing the eyes to bulge outwards.
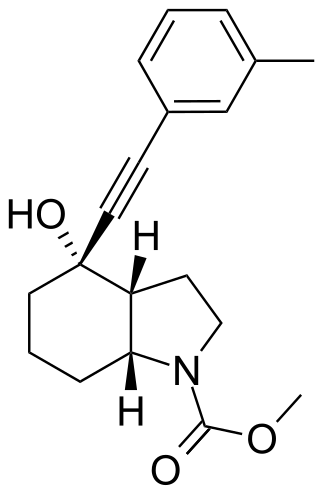
Mavoglurant (developmental code name AFQ-056) is an experimental drug candidate for the treatment of fragile X syndrome and other conditions. It exerts its effect as an antagonist of the metabotropic glutamate receptor 5 (mGluR5).
Calcitonin gene-related peptide (CGRP) receptor antagonists are a class of drugs that act as antagonists of the calcitonin gene-related peptide receptor (CGRPR).

Paritaprevir is an acylsulfonamide inhibitor of the NS3-4A serine protease manufactured by Abbott Laboratories that shows promising results as a treatment of hepatitis C. When given in combination with ritonavir and ribavirin for 12 weeks, the rate of sustained virologic response at 24 weeks after treatment has been estimated to be 95% for those with hepatitis C virus genotype 1. Resistance to treatment with paritaprevir is uncommon, because it targets the binding site, but has been seen to arise due to mutations at positions 155 and 168 in NS3.

Ozanimod, sold under the brand name Zeposia, is an immunomodulatory medication for the treatment of relapsing multiple sclerosis and ulcerative colitis. It acts as a sphingosine-1-phosphate receptor (S1PR) agonist, sequestering lymphocytes to peripheral lymphoid organs and away from their sites of chronic inflammation.














