
F. Hoffmann-La Roche AG, commonly known as Roche, is a Swiss multinational holding healthcare company that operates worldwide under two divisions: Pharmaceuticals and Diagnostics. Its holding company, Roche Holding AG, has shares listed on the SIX Swiss Exchange. The company headquarters are located in Basel. Roche is the fifth-largest pharmaceutical company in the world by revenue and the leading provider of cancer treatments globally. In 2023, the company’s seat in Forbes Global 2000 was 76.

Trastuzumab, sold under the brand name Herceptin among others, is a monoclonal antibody used to treat breast cancer and stomach cancer. It is specifically used for cancer that is HER2 receptor positive. It may be used by itself or together with other chemotherapy medication. Trastuzumab is given by slow injection into a vein and injection just under the skin.
Bevacizumab, sold under the brand name Avastin among others, is a monoclonal antibody medication used to treat a number of types of cancers and a specific eye disease. For cancer, it is given by slow injection into a vein (intravenous) and used for colon cancer, lung cancer, ovarian cancer, glioblastoma, and renal-cell carcinoma. In many of these diseases it is used as a first-line therapy. For age-related macular degeneration it is given by injection into the eye (intravitreal).

Daiichi Sankyo Company, Limited is a global pharmaceutical company and the second-largest pharmaceutical company in Japan. It achieved JPY 1,278 billion in revenue in 2022. The company owns the American pharmaceutical company American Regent.

Pertuzumab, sold under the brand name Perjeta, is a monoclonal antibody used in combination with trastuzumab and docetaxel for the treatment of metastatic HER2-positive breast cancer; it also used in the same combination as a neoadjuvant in early HER2-positive breast cancer.
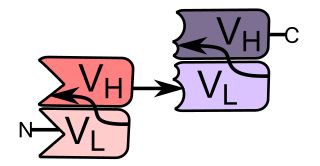
Bi-specific T-cell engagers (BiTEs) are a class of artificial bispecific monoclonal antibodies that are investigated for use as anti-cancer drugs. They direct a host's immune system, more specifically the T cells' cytotoxic activity, against cancer cells. BiTE is a registered trademark of Micromet AG.
Obinutuzumab, sold under the brand name Gazyva among others, is a humanized anti-CD20 monoclonal antibody used as a treatment for cancer. It was originated by GlycArt Biotechnology AG and developed by Roche.

Antibody–drug conjugates or ADCs are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer. Unlike chemotherapy, ADCs are intended to target and kill tumor cells while sparing healthy cells. As of 2019, some 56 pharmaceutical companies were developing ADCs.

Trastuzumab emtansine, sold under the brand name Kadcyla, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the cytotoxic agent DM1. Trastuzumab alone stops growth of cancer cells by binding to the HER2 receptor, whereas trastuzumab emtansine undergoes receptor-mediated internalization into cells, is catabolized in lysosomes where DM1-containing catabolites are released and subsequently bind tubulin to cause mitotic arrest and cell death. Trastuzumab binding to HER2 prevents homodimerization or heterodimerization (HER2/HER3) of the receptor, ultimately inhibiting the activation of MAPK and PI3K/AKT cellular signalling pathways. Because the monoclonal antibody targets HER2, and HER2 is only over-expressed in cancer cells, the conjugate delivers the cytotoxic agent DM1 specifically to tumor cells. The conjugate is abbreviated T-DM1.

Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.
Margetuximab, sold under the brand name Margenza, is a chimeric IgG monoclonal antibody medication against HER2 used for the treatment of cancer.
ImmunoGen, Inc. was a biotechnology company focused on the development of antibody-drug conjugate (ADC) therapeutics for the treatment of cancer. ImmunoGen was founded in 1981 and was headquartered in Waltham, Massachusetts.
Celltrion, Inc. is a biopharmaceutical company headquartered in Incheon, South Korea. Celltrion Healthcare conducts worldwide marketing, sales, and distribution of biological medicines developed by Celltrion. Celltrion's founder, Seo Jung-jin, is the richest person in South Korea.

Atezolizumab, sold under the brand name Tecentriq among others, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma and alveolar soft part sarcoma, but discontinued for use in triple-negative breast cancer (TNBC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).

Sacituzumab govitecan, sold under the brand name Trodelvy by Gilead Sciences, is a Trop-2-directed antibody and topoisomerase inhibitor drug conjugate used for the treatment of metastatic triple-negative breast cancer and metastatic urothelial cancer.
PDL BioPharma is a publicly traded American holding company that since 2008 manages patents and other intellectual property that had been generated by the company. In 2008 in response to shareholder pressure, PDL spun out its active development programs to a company called Facet Biotech that it capitalized with $400 million.
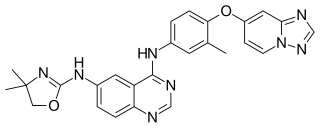
Tucatinib, sold under the brand name Tukysa, is an anticancer medication used for the treatment of HER2-positive breast cancer. It is a small molecule inhibitor of HER2. It was developed by Array BioPharma and licensed to Cascadian Therapeutics.
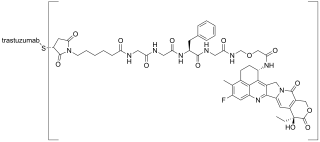
Trastuzumab deruxtecan, sold under the brand name Enhertu, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the topoisomerase I inhibitor deruxtecan. It is licensed for the treatment of breast cancer or gastric or gastroesophageal adenocarcinoma. Trastuzumab binds to and blocks signaling through epidermal growth factor receptor 2 (HER2/neu) on cancers that rely on it for growth. Additionally, once bound to HER2 receptors, the antibody is internalized by the cell, carrying the bound deruxtecan along with it, where it interferes with the cell's ability to make DNA structural changes and replicate its DNA during cell division, leading to DNA damage when the cell attempts to replicate itself, destroying the cell.
Pertuzumab/trastuzumab/hyaluronidase, sold under the brand name Phesgo, is a fixed-dose combination medication to treat adults with HER2-positive breast cancer that has spread to other parts of the body, and for treatment of adults with early HER2-positive breast cancer. It contains pertuzumab, trastuzumab, and hyaluronidase–zzxf. It is injected under the skin via subcutaneous injection in the thigh. In the European Union, Phesgo contains the active ingredients pertuzumab and trastuzumab along with the enzyme vorhyaluronidase alfa.
Trastuzumab/hyaluronidase, sold under the brand name Herceptin SC among others, is a fixed-dose combination medication for the treatment of HER2-overexpressing breast cancer in adults. It is a combination of trastuzumab and hyaluronidase.












