The HIV Prevention Trials Network (HPTN) is a worldwide collaborative clinical trials network that brings together investigators, ethicists, community and other partners to develop and test the safety and efficacy of interventions designed to prevent the acquisition and transmission of HIV. HPTN studies evaluate new HIV prevention interventions and strategies in populations and geographical regions that bear a disproportionate burden of infection. The HPTN is committed to the highest ethical standards for its clinical trials and recognizes the importance of community engagement in all phases of the research process.
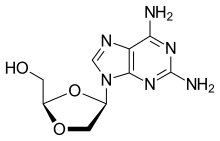
Vicriviroc, previously named SCH 417690 and SCH-D, is a pyrimidine CCR5 entry inhibitor of HIV-1. It was developed by the pharmaceutical company Schering-Plough. Merck decided to not pursue regulatory approval for use in treatment-experienced patients because the drug did not meet primary efficacy endpoints in late stage trials. Clinical trials continue in patients previously untreated for HIV.
Naproxcinod (nitronaproxen) is a nonsteroidal anti-inflammatory drug (NSAID) developed by the French pharmaceutical company NicOx. It is a derivative of naproxen with a nitroxybutyl ester to allow it to also act as a nitric oxide (NO) donor. This second mechanism of action makes naproxcinod the first in a new class of drugs, the cyclooxygenase inhibiting nitric oxide donators (CINODs), that are hoped to produce similar analgesic efficacy to traditional NSAIDs, but with less gastrointestinal and cardiovascular side effects.
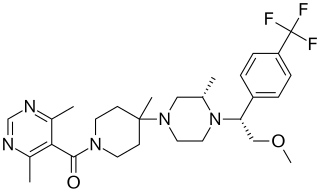
Leronlimab is a humanized monoclonal antibody targeted against the CCR5 receptor found on T lymphocytes of the human immune system. It is being investigated as a potential therapy in the treatment of COVID-19, triple negative breast cancer, and HIV infection. The United States Food and Drug Administration has designated PRO 140 for fast-track approval. In February 2008, the drug entered Phase 2 clinical trials and a phase 3 trial was begun in 2015. In February 2018, Cytodyn Inc reported that the primary endpoint had been achieved in the PRO 140 pivotal combination therapy trial in HIV infection. In 2020 CytoDyn submitted a fast-track biologics license application for treatment of CCR5-tropic HIV-1 Infection.

NS-2359 (GSK-372,475) is a serotonin-norepinephrine-dopamine reuptake inhibitor. It was under development by GlaxoSmithKline (GSK) as an antidepressant, but was discontinued in 2009 when phase II clinical trials showed the drug was not effective and not well tolerated. The results did not support further effort by the company. NS-2359 was also in clinical trials for the treatment of ADHD, phase II having been completed in 2007. A phase I clinical trial exploring the effect of NS-2359 on cocaine-dependent individuals was completed in 2002.

Riociguat, sold under the brand name Adempas, is a medication by Bayer that is a stimulator of soluble guanylate cyclase (sGC). It is used to treat two forms of pulmonary hypertension (PH): chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension (PAH). Riociguat constitutes the first drug of the class of sGC stimulators. The drug has a half-life of 12 hours and will decrease dyspnea associated with pulmonary arterial hypertension.

Fosbretabulin is a microtubule destabilizing experimental drug, a type of vascular-targeting agent, a drug designed to damage the vasculature of cancer tumours causing central necrosis. It is a derivative of combretastatin. It is formulated as the salts fosbretabulin disodium and fosbretabulin tromethamine.

Losmapimod (GW856553X) is an investigational drug being developed by Fulcrum Therapeutics for the treatment of facioscapulohumeral muscular dystrophy (FSHD); a phase III clinical trial is pending approval. Losmapimod selectively inhibits enzymes p38α/β mitogen-activated protein kinases (MAPKs), which are modulators of DUX4 expression and mediators of inflammation.
Briakinumab (ABT-874) is a human monoclonal antibody being developed by Abbott Laboratories for the treatment of rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis. As of 2011 drug development for psoriasis has been discontinued in the U.S. and Europe.

Phosphoinositide 3-kinase inhibitors are a class of medical drugs that are mainly used to treat advanced cancers. They function by inhibiting one or more of the phosphoinositide 3-kinase (PI3K) enzymes, which are part of the PI3K/AKT/mTOR pathway. This signal pathway regulates cellular functions such as growth and survival. It is strictly regulated in healthy cells, but is always active in many cancer cells, allowing the cancer cells to better survive and multiply. PI3K inhibitors block the PI3K/AKT/mTOR pathway and thus slow down cancer growth. They are examples of a targeted therapy. While PI3K inhibitors are an effective treatment, they can have very severe side effects and are therefore only used if other treatments have failed or are not suitable.
Pegsunercept is a drug for the treatment of rheumatoid arthritis. As of January 2010, Phase II clinical trials have been completed. It is being developed by Amgen.
Amatuximab is a chimeric monoclonal antibody designed for the treatment of cancer. It was developed by Morphotek, Inc.
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.

Brexpiprazole, sold under the brand name Rexulti among others, is a medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer's disease. It is an atypical antipsychotic.

Filgotinib, sold under the brand name Jyseleca, is a medication used for the treatment of rheumatoid arthritis (RA). It was developed by the Belgian-Dutch biotech company Galapagos NV.
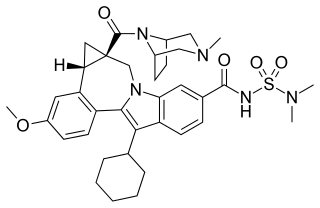
Beclabuvir is an antiviral drug for the treatment of hepatitis C virus (HCV) infection that has been studied in clinical trials. In February 2017, Bristol-Myers Squibb began sponsoring a post-marketing trial of beclabuvir, in combination with asunaprevir and daclatasvir, to study the combination's safety profile with regard to liver function. From February 2014 to November 2016, a phase II clinical trial was conducted on the combination of asunaprevir/daclatasvir/beclabuvir on patients infected with both HIV and HCV. Furthermore, a recent meta-analysis of six published six clinical trials showed high response rates in HCV genotype 1-infected patients treated with daclatasvir, asunaprevir, and beclabuvir irrespective of ribavirin use, prior interferon-based therapy, or restriction on noncirrhotic patients, IL28B genotype, or baseline resistance-associated variants
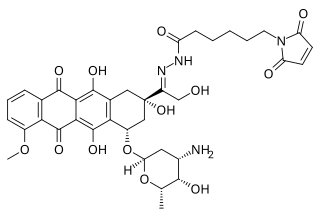
Aldoxorubicin (INNO-206) is a tumor-targeted doxorubicin conjugate in development by CytRx. Specifically, it is the (6-maleimidocaproyl) hydrazone of doxorubicin. Essentially, this chemical name describes doxorubicin attached to an acid-sensitive linker.
Opicinumab (BIIB033) is a fully human monoclonal antibody designed for the treatment of multiple sclerosis, acute optic neuritis (AON), and other associated demyelinating diseases. A biologic drug, it is designed to function as a LINGO-1 protein antagonist, known as "Anti-Lingo-1".
Emapalumab, sold under the brand name Gamifant, is an anti-interferon-gamma (IFNγ) antibody medication used for the treatment of hemophagocytic lymphohistiocytosis (HLH), which has no cure.

Upadacitinib, sold under the brand name Rinvoq, is a medication used for the treatment of rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, ulcerative colitis, Crohn's disease, ankylosing spondylitis, and axial spondyloarthritis. Upadacitinib is a Janus kinase (JAK) inhibitor that works by blocking the action of enzymes called Janus kinases. These enzymes are involved in setting up processes that lead to inflammation, and blocking their effect brings inflammation in the joints under control.